Temporal and fluoride control of secondary metabolism regulates cellular organofluorine biosynthesis
- PMID: 22769062
- PMCID: PMC5818262
- DOI: 10.1021/cb3002057
Temporal and fluoride control of secondary metabolism regulates cellular organofluorine biosynthesis
Abstract
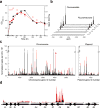
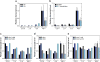
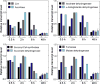
Elucidating mechanisms of natural organofluorine biosynthesis is essential for a basic understanding of fluorine biochemistry in living systems as well as for expanding biological methods for fluorine incorporation into small molecules of interest. To meet this goal we have combined massively parallel sequencing technologies, genetic knockout, and in vitro biochemical approaches to investigate the fluoride response of the only known genetic host of an organofluorine-producing pathway, Streptomyces cattleya. Interestingly, we have discovered that the major mode of S. cattleya's resistance to the fluorinated toxin it produces, fluoroacetate, may be due to temporal control of production rather than the ability of the host's metabolic machinery to discriminate between fluorinated and non-fluorinated molecules. Indeed, neither the acetate kinase/phosphotransacetylase acetate assimilation pathway nor the TCA cycle enzymes (citrate synthase and aconitase) exclude fluorinated substrates based on in vitro biochemical characterization. Furthermore, disruption of the fluoroacetate resistance gene encoding a fluoroacetyl-CoA thioesterase (FlK) does not appear to lead to an observable growth defect related to organofluorine production. By showing that a switch in central metabolism can mediate and control molecular fluorine incorporation, our findings reveal a new potential strategy toward diversifying simple fluorinated building blocks into more complex products.
Figures


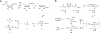
Similar articles
-
The rare fluorinated natural products and biotechnological prospects for fluorine enzymology.Methods Enzymol. 2012;516:219-35. doi: 10.1016/B978-0-12-394291-3.00003-4. Methods Enzymol. 2012. PMID: 23034231 Review.
-
Entropy drives selective fluorine recognition in the fluoroacetyl-CoA thioesterase from Streptomyces cattleya.Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):E2193-E2201. doi: 10.1073/pnas.1717077115. Epub 2018 Feb 16. Proc Natl Acad Sci U S A. 2018. PMID: 29453276 Free PMC article.
-
Structural and biochemical studies of a fluoroacetyl-CoA-specific thioesterase reveal a molecular basis for fluorine selectivity.Biochemistry. 2010 Nov 2;49(43):9269-79. doi: 10.1021/bi101102u. Biochemistry. 2010. PMID: 20836570 Free PMC article.
-
The gene cluster for fluorometabolite biosynthesis in Streptomyces cattleya: a thioesterase confers resistance to fluoroacetyl-coenzyme A.Chem Biol. 2006 May;13(5):475-84. doi: 10.1016/j.chembiol.2006.02.014. Chem Biol. 2006. PMID: 16720268
-
Fluorometabolite biosynthesis and the fluorinase from Streptomyces cattleya.Nat Prod Rep. 2004 Dec;21(6):773-84. doi: 10.1039/b415087m. Epub 2004 Oct 28. Nat Prod Rep. 2004. PMID: 15565254 Review.
Cited by
-
Biosynthesis of Fluorinated Peptaibols Using a Site-Directed Building Block Incorporation Approach.J Nat Prod. 2017 Jun 23;80(6):1883-1892. doi: 10.1021/acs.jnatprod.7b00189. Epub 2017 Jun 8. J Nat Prod. 2017. PMID: 28594169 Free PMC article.
-
Synthetic biology approaches to fluorinated polyketides.Acc Chem Res. 2015 Mar 17;48(3):584-92. doi: 10.1021/ar500415c. Epub 2015 Feb 26. Acc Chem Res. 2015. PMID: 25719427 Free PMC article.
-
Molecular recognition of fluorine impacts substrate selectivity in the fluoroacetyl-CoA thioesterase FlK.Biochemistry. 2014 Apr 1;53(12):2053-63. doi: 10.1021/bi4015049. Epub 2014 Mar 17. Biochemistry. 2014. PMID: 24635371 Free PMC article.
-
The link between ancient microbial fluoride resistance mechanisms and bioengineering organofluorine degradation or synthesis.Nat Commun. 2024 May 30;15(1):4593. doi: 10.1038/s41467-024-49018-1. Nat Commun. 2024. PMID: 38816380 Free PMC article. Review.
-
Expanding the fluorine chemistry of living systems using engineered polyketide synthase pathways.Science. 2013 Sep 6;341(6150):1089-94. doi: 10.1126/science.1242345. Science. 2013. PMID: 24009388 Free PMC article.
References
-
- O’Hagan D. Recent developments on the fluorinase from Streptomyces cattleya. J Fluor Chem. 2006;127:1479–1483.
-
- Sanada M, Miyano T, Iwadare S, Williamson J, Arison B, Smith J, Douglas A, Liesch J, Inamine E. Biosynthesis of fluorothreonine and fluoroacetic acid by the thienamycin producer, Streptomyces cattleya. J Antibiot. 1986;39:259–265. - PubMed
-
- Gribble GW. The diversity of naturally produced organohalogens. Chemosphere. 2003;52:289–297. - PubMed
-
- Gribble GW. The Handbook of Environmental Chemistry. Springer-Verlag; Berlin: 2002. pp. 121–136.
-
- Fukuda K, Tamura T, Segawa Y, Mutaguchi Y, Inagaki K. Enhanced production of the fluorinated nucleoside antibiotic nucleocidin by a rifR-resistant mutant of Streptomyces calvus IFO13200. Actinomycetologica. 2009;23:51–55.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

