Dose-dependent expression of neuronal injury markers during experimental osteoarthritis induced by monoiodoacetate in the rat
- PMID: 22769424
- PMCID: PMC3495674
- DOI: 10.1186/1744-8069-8-50
Dose-dependent expression of neuronal injury markers during experimental osteoarthritis induced by monoiodoacetate in the rat
Abstract
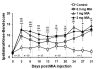
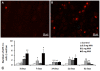
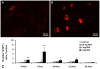
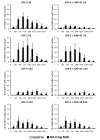
Background: It was recently reported that the mono-iodoacetate (MIA) experimental model of osteoarthritis (OA) courses with changes of neurons innervating the affected joints that are commonly interpreted as a neuronal response to axonal injury. To better characterize these changes, we evaluated the expression of two markers of neuronal damage, ATF-3 and NPY, and the growth associated protein GAP-43, in primary afferent neurons of OA animals injected with three different doses of MIA (0.3, 1 or 2 mg). Measurements were performed at days 3, 7, 14, 21 and 31 post-MIA injection.
Results: OA animals showed the characteristic histopathological changes of the joints and the accompanying nociceptive behaviour, evaluated by the Knee-Bed and CatWalk tests. An increase of ATF-3 expression was detected in the DRG of OA animals as early as 3 days after the injection of 1 or 2 mg of MIA and 7 days after the injection of 0.3 mg. NPY expression was increased in animals injected with 1 or 2 mg of MIA, at day 3 or in all time-points, respectively. From day 7 onwards there was a massive increase of GAP-43 expression in ATF-3 cells.
Conclusions: The expression of the neuronal injury markers ATF-3 and NPY as well as an up-regulation of GAP-43 expression, indicative of peripheral fibre regeneration, suggests that axonal injury and a regeneration response may be happening in this model of OA. This opens new perspectives in the unravelling of the physiopathology of the human disease.
Figures



References
-
- Hart FD. Pain in osteoarthrosis. Practitioner. 1974;212:244–250. - PubMed
-
- Schaible HG, Schmidt RF. Responses of fine medial articular nerve afferents to passive movements of knee joints. J Neurophysiol. 1983;49:1118–1126. - PubMed
-
- Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003;31:619–624. - PubMed
-
- Kalbhen DA. Chemical model of osteoarthritis – a pharmacological evaluation. J Rheumatol. 1987;14:130–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

