Mechanism of Cd2+ coordination during slow inactivation in potassium channels
- PMID: 22771214
- PMCID: PMC3846092
- DOI: 10.1016/j.str.2012.03.027
Mechanism of Cd2+ coordination during slow inactivation in potassium channels
Abstract
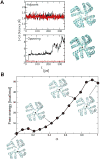
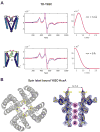
In K+ channels, rearrangements of the pore outer vestibule have been associated with C-type inactivation gating. Paradoxically, the crystal structure of Open/C-type inactivated KcsA suggests these movements to be modest in magnitude. In this study, we show that under physiological conditions, the KcsA outer vestibule undergoes relatively large dynamic rearrangements upon inactivation. External Cd2+ enhances the rate of C-type inactivation in an cysteine mutant (Y82C) via metal-bridge formation. This effect is not present in a non-inactivating mutant (E71A/Y82C). Tandem dimer and tandem tetramer constructs of equivalent cysteine mutants in KcsA and Shaker K+ channels demonstrate that these Cd2+ metal bridges are formed only between adjacent subunits. This is well supported by molecular dynamics simulations. Based on the crystal structure of Cd2+ -bound Y82C-KcsA in the closed state, together with electron paramagnetic resonance distance measurements in the KcsA outer vestibule, we suggest that subunits must dynamically come in close proximity as the channels undergo inactivation.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Structural basis for the coupling between activation and inactivation gates in K(+) channels.Nature. 2010 Jul 8;466(7303):272-5. doi: 10.1038/nature09136. Nature. 2010. PMID: 20613845 Free PMC article.
-
Molecular determinants of gating at the potassium-channel selectivity filter.Nat Struct Mol Biol. 2006 Apr;13(4):311-8. doi: 10.1038/nsmb1069. Epub 2006 Mar 12. Nat Struct Mol Biol. 2006. PMID: 16532009
-
Dynamics transitions at the outer vestibule of the KcsA potassium channel during gating.Proc Natl Acad Sci U S A. 2014 Feb 4;111(5):1831-6. doi: 10.1073/pnas.1314875111. Epub 2014 Jan 15. Proc Natl Acad Sci U S A. 2014. PMID: 24429344 Free PMC article.
-
EPR approaches to ion channel structure and function.Novartis Found Symp. 2002;245:146-58; discussion 158-64, 165-8. doi: 10.1002/0470868759.ch10. Novartis Found Symp. 2002. PMID: 12027005 Review.
-
Diverse gating in K+ channels: differential role of the pore-helix glutamate in stabilizing the channel pore.Biochem Biophys Res Commun. 2011 Sep 16;413(1):1-4. doi: 10.1016/j.bbrc.2011.08.062. Epub 2011 Aug 22. Biochem Biophys Res Commun. 2011. PMID: 21872570 Review.
Cited by
-
A facile approach for the in vitro assembly of multimeric membrane transport proteins.Elife. 2018 Jun 11;7:e36478. doi: 10.7554/eLife.36478. Elife. 2018. PMID: 29889023 Free PMC article.
-
Gating-related Structural Dynamics of the MgtE Magnesium Channel in Membrane-Mimetics Utilizing Site-Directed Tryptophan Fluorescence.J Mol Biol. 2021 Aug 20;433(17):166691. doi: 10.1016/j.jmb.2020.10.025. Epub 2020 Oct 22. J Mol Biol. 2021. PMID: 33203509 Free PMC article.
-
Structural Dynamics of the Paddle Motif Loop in the Activated Conformation of KvAP Voltage Sensor.Biophys J. 2020 Feb 25;118(4):873-884. doi: 10.1016/j.bpj.2019.08.017. Epub 2019 Aug 22. Biophys J. 2020. PMID: 31547975 Free PMC article.
-
Conformational plasticity in the KcsA potassium channel pore helix revealed by homo-FRET studies.Sci Rep. 2019 Apr 17;9(1):6215. doi: 10.1038/s41598-019-42405-5. Sci Rep. 2019. PMID: 30996281 Free PMC article.
-
C-type inactivation of voltage-gated K+ channels: pore constriction or dilation?J Gen Physiol. 2013 Feb;141(2):151-60. doi: 10.1085/jgp.201210888. Epub 2013 Jan 14. J Gen Physiol. 2013. PMID: 23319730 Free PMC article. No abstract available.
References
-
- Ader C, et al. A structural link between inactivation and block of a K+ channel. Nat Struct Mol Biol. 2008;15:605–612. - PubMed
-
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
-
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

