Psychiatric drugs bind to classical targets within early exocytotic pathways: therapeutic effects
- PMID: 22771239
- PMCID: PMC6167061
- DOI: 10.1016/j.biopsych.2012.05.020
Psychiatric drugs bind to classical targets within early exocytotic pathways: therapeutic effects
Abstract
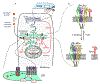
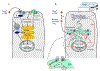
The classical targets for antipsychotic and antidepressant drugs are G protein-coupled receptors and neurotransmitter transporters, respectively. Full therapeutic actions of these drugs require several weeks. We show how therapeutic effects may eventually accrue after existing therapeutic ligands bind to these classical targets, not on the plasma membrane but rather within endoplasmic reticulum (ER) and cis-Golgi. Consequences of such binding may include pharmacological chaperoning: the nascent drug targets are stabilized against degradation and can therefore exit the ER more readily. Another effect may be matchmaking: heterodimers and homodimers of the target form and can more readily exit the ER. Summarizing recent data for nicotinic receptors, we explain how such effects could lead to reduced ER stress and to a decreased unfolded protein response, including changes in gene activation and protein synthesis. In effects not directly related to cellular stress, escorting would allow increased ER exit and trafficking of known associated proteins, as well as other proteins such as growth factors and their receptors, producing both cell-autonomous and non-cell-autonomous effects. Axonal transport of relevant proteins may underlie the several weeks required for full therapy. In contrast, the antidepressant effects of ketamine and other N-methyl-D-aspartate receptor ligands, which occur within <2 hours, could arise from dendritically localized intracellular binding, followed by chaperoning, matchmaking, escorting, and reduced ER stress. Thus, the effects of intracellular binding extend beyond proteostasis of the targets themselves and involve pathways distinct from ion channel and G protein activation. We propose experimental tests and note pathophysiological correlates.
Copyright © 2012 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Routledge KE, Gupta V, Balch WE (2010): Emergent properties of proteostasis-COPII coupled systems in human health and disease. Mol Membr Biol 27:385–397. - PubMed
-
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ (2005): Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron 46:595–607. - PubMed
-
- Kuryatov A, Luo J, Cooper J, Lindstrom J (2005): Nicotine acts as a pharmacological chaperone to up-regulate human α4β2 acetylcholine receptors. Mol Pharmacol 68:1839–1851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM-081662/GM/NIGMS NIH HHS/United States
- R01 AG033954/AG/NIA NIH HHS/United States
- RC1 MH088550/MH/NIMH NIH HHS/United States
- R01 DA017279/DA/NIDA NIH HHS/United States
- P50 MH086383/MH/NIMH NIH HHS/United States
- DA-09121/DA/NIDA NIH HHS/United States
- DA-17279/DA/NIDA NIH HHS/United States
- DA-19375/DA/NIDA NIH HHS/United States
- AG-33954/AG/NIA NIH HHS/United States
- MH-086383/MH/NIMH NIH HHS/United States
- R01 GM081662/GM/NIGMS NIH HHS/United States
- R01 DA009121/DA/NIDA NIH HHS/United States
- GM-29836/GM/NIGMS NIH HHS/United States
- MH-088550/MH/NIMH NIH HHS/United States
- U19 DA019375/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

