Cell replacement and visual restoration by retinal sheet transplants
- PMID: 22771454
- PMCID: PMC3472113
- DOI: 10.1016/j.preteyeres.2012.06.003
Cell replacement and visual restoration by retinal sheet transplants
Abstract
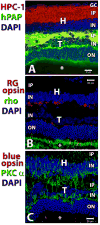
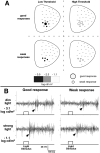
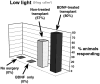
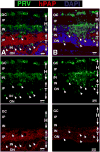
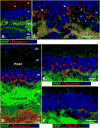
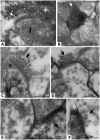
Retinal diseases such as age-related macular degeneration (ARMD) and retinitis pigmentosa (RP) affect millions of people. Replacing lost cells with new cells that connect with the still functional part of the host retina might repair a degenerating retina and restore eyesight to an unknown extent. A unique model, subretinal transplantation of freshly dissected sheets of fetal-derived retinal progenitor cells, combined with its retinal pigment epithelium (RPE), has demonstrated successful results in both animals and humans. Most other approaches are restricted to rescue endogenous retinal cells of the recipient in earlier disease stages by a 'nursing' role of the implanted cells and are not aimed at neural retinal cell replacement. Sheet transplants restore lost visual responses in several retinal degeneration models in the superior colliculus (SC) corresponding to the location of the transplant in the retina. They do not simply preserve visual performance - they increase visual responsiveness to light. Restoration of visual responses in the SC can be directly traced to neural cells in the transplant, demonstrating that synaptic connections between transplant and host contribute to the visual improvement. Transplant processes invade the inner plexiform layer of the host retina and form synapses with presumable host cells. In a Phase II trial of RP and ARMD patients, transplants of retina together with its RPE improved visual acuity. In summary, retinal progenitor sheet transplantation provides an excellent model to answer questions about how to repair and restore function of a degenerating retina. Supply of fetal donor tissue will always be limited but the model can set a standard and provide an informative base for optimal cell replacement therapies such as embryonic stem cell (ESC)-derived therapy.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Progress in retinal sheet transplantation.Prog Retin Eye Res. 2004 Sep;23(5):475-94. doi: 10.1016/j.preteyeres.2004.05.003. Prog Retin Eye Res. 2004. PMID: 15302347 Review.
-
Retinal transplantation--advantages of intact fetal sheets.Prog Retin Eye Res. 2002 Jan;21(1):57-73. doi: 10.1016/s1350-9462(01)00020-9. Prog Retin Eye Res. 2002. PMID: 11906811 Review.
-
Transplantation of neuroblastic progenitor cells as a sheet preserves and restores retinal function.Semin Ophthalmol. 2005 Jan-Mar;20(1):31-42. doi: 10.1080/08820530590921873. Semin Ophthalmol. 2005. PMID: 15804842 Review.
-
Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets.Eur J Neurosci. 2010 Feb;31(3):508-20. doi: 10.1111/j.1460-9568.2010.07085.x. Epub 2010 Jan 25. Eur J Neurosci. 2010. PMID: 20105230 Free PMC article.
-
Vision change after sheet transplant of fetal retina with retinal pigment epithelium to a patient with retinitis pigmentosa.Arch Ophthalmol. 2004 Aug;122(8):1159-65. doi: 10.1001/archopht.122.8.1159. Arch Ophthalmol. 2004. PMID: 15302656
Cited by
-
Self-organization, quality control, and preclinical studies of human iPSC-derived retinal sheets for tissue-transplantation therapy.Commun Biol. 2023 Feb 10;6(1):164. doi: 10.1038/s42003-023-04543-5. Commun Biol. 2023. PMID: 36765170 Free PMC article.
-
Retinal organoids on-a-chip: a micro-millifluidic bioreactor for long-term organoid maintenance.Lab Chip. 2021 Sep 7;21(17):3361-3377. doi: 10.1039/d1lc00011j. Epub 2021 Jul 8. Lab Chip. 2021. PMID: 34236056 Free PMC article.
-
Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration.Proc Natl Acad Sci U S A. 2016 Jan 5;113(1):E81-90. doi: 10.1073/pnas.1512590113. Epub 2015 Dec 22. Proc Natl Acad Sci U S A. 2016. PMID: 26699487 Free PMC article.
-
In vitro formation of neuroclusters in microfluidic devices and cell migration as a function of stromal-derived growth factor 1 gradients.Cell Adh Migr. 2017 Jan 2;11(1):1-12. doi: 10.1080/19336918.2015.1131388. Epub 2016 Jan 8. Cell Adh Migr. 2017. PMID: 26744909 Free PMC article.
-
Challenges and Advances in the Production of Transplantable Retinal Tissue from Retinal Organoids.J Ophthalmic Vis Res. 2025 Jun 18;20:10.18502/jovr.v20.17499. doi: 10.18502/jovr.v20.17499. eCollection 2025. J Ophthalmic Vis Res. 2025. PMID: 40689116 Free PMC article.
References
-
- Abe T, Tomita H, Kano T, Yoshida M, Ohashi T, Nakamura Y, Nishikawa S, Tamai M. Autologous iris pigment epithelial cell transplantation in monkey subretinal region. Curr Eye Res. 2000a;20:268–275. - PubMed
-
- Abe T, Yoshida M, Tomita H, Kano T, Sato M, Wada Y, Fuse N, Yamada T, Tamai M. Auto iris pigment epithelial cell transplantation in patients with age-related macular degeneration: short-term results. Tohoku J Exp Med. 2000b;191:7–20. - PubMed
-
- Abe T, Yoshida M, Yoshioka Y, Wakusawa R, Tokita-Ishikawa Y, Seto H, Tamai M, Nishida K. Iris pigment epithelial cell transplantation for degenerative retinal diseases. Prog Retin Eye Res. 2007;26:302–321. - PubMed
-
- Aisenbrey S, Lafaut BA, Szurman P, Hilgers RD, Esser P, Walter P, Bartz-Schmidt KU, Thumann G. Iris pigment epithelial translocation in the treatment of exudative macular degeneration: a 3-year follow-up. Arch Ophthalmol. 2006;124:183–188. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

