Strain-specific colonization patterns and serum modulation of multi-species oral biofilm development
- PMID: 22771792
- PMCID: PMC3410037
- DOI: 10.1016/j.anaerobe.2012.06.003
Strain-specific colonization patterns and serum modulation of multi-species oral biofilm development
Abstract
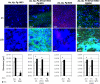
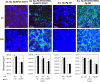
Periodontitis results from an ecological shift in the composition of subgingival biofilms. Subgingival community maturation is modulated by inter-organismal interactions and the relationship of communities with the host. In an effort to better understand this process, we evaluated biofilm formation, with oral commensal species, by three strains of the subgingivally prevalent microorganism Fusobacterium nucleatum and four strains of the periodontopathogen Porphyromonas gingivalis. We also tested the effect of serum, which resembles gingival exudates, on subgingival biofilms. Biofilms were allowed to develop in flow cells using salivary medium. We found that although not all strains of F. nucleatum were able to grow in mono-species biofilms, forming a community with health-associated partners Actinomyces oris and Veillonella parvula promoted biofilm growth of all F. nucleatum strains. Strains of P. gingivalis also showed variable ability to form mono-species biofilms. P. gingivalis W50 and W83 did not form biofilms, while ATCC 33277 and 381 formed biofilm structures, but only strain ATCC 33277 grew over time. Unlike the enhanced growth of F. nucleatum with the two health-associated species, no strain of P. gingivalis grew in three-species communities with A. oris and V. parvula. However, addition of F. nucleatum facilitated growth of P. gingivalis ATCC 33277 with health-associated partners. Importantly, serum negatively affected the adhesion of F. nucleatum, while it favored biofilm growth by P. gingivalis. This work highlights strain specificity in subgingival biofilm formation. Environmental factors such as serum alter the colonization patterns of oral microorganisms and could impact subgingival biofilms by selectively promoting pathogenic species.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

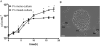



References
-
- Kolenbrander PE. Surface recognition among oral bacteria: multigeneric coaggregations and their mediators. Crit Rev Microbiol. 1989;17:137–59. - PubMed
-
- Bradshaw DJ, Homer KA, Marsh PD, Beighton D. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology. 1994;140(Pt 12):3407–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

