Live imaging using adaptive optics with fluorescent protein guide-stars
- PMID: 22772285
- PMCID: PMC3601654
- DOI: 10.1364/OE.20.015969
Live imaging using adaptive optics with fluorescent protein guide-stars
Abstract
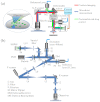
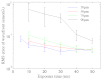
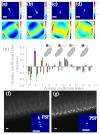
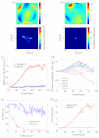
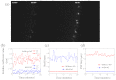
Spatially and temporally dependent optical aberrations induced by the inhomogeneous refractive index of live samples limit the resolution of live dynamic imaging. We introduce an adaptive optical microscope with a direct wavefront sensing method using a Shack-Hartmann wavefront sensor and fluorescent protein guide-stars for live imaging. The results of imaging Drosophila embryos demonstrate its ability to correct aberrations and achieve near diffraction limited images of medial sections of large Drosophila embryos. GFP-polo labeled centrosomes can be observed clearly after correction but cannot be observed before correction. Four dimensional time lapse images are achieved with the correction of dynamic aberrations. These studies also demonstrate that the GFP-tagged centrosome proteins, Polo and Cnn, serve as excellent biological guide-stars for adaptive optics based microscopy.
Figures

References
-
- Booth M. J., “Adaptive optics in microscopy,” Phil. Trans. R. Soc. A–Math. Phys. Eng. Sci. 365, 2829–2843 (2007). - PubMed
-
- R. K. Tyson, Principles of Adaptive Optics (Academic, 1991).
-
- J. Porter, H. Queener, J. Lin, K. Thorn, and A. A. S. Awwal, Adaptive Optics for Vision Science: Principles, Practices, Design and Applications, (Wiley, 2006).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

