The contribution of cellular mechanotransduction to cardiomyocyte form and function
- PMID: 22772714
- PMCID: PMC3786397
- DOI: 10.1007/s10237-012-0419-2
The contribution of cellular mechanotransduction to cardiomyocyte form and function
Abstract
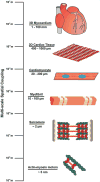
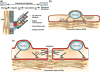
Myocardial development is regulated by an elegantly choreographed ensemble of signaling events mediated by a multitude of intermediates that take a variety of forms. Cellular differentiation and maturation are a subset of vertically integrated processes that extend over several spatial and temporal scales to create a well-defined collective of cells that are able to function cooperatively and reliably at the organ level. Early efforts to understand the molecular mechanisms of cardiomyocyte fate determination focused primarily on genetic and chemical mediators of this process. However, increasing evidence suggests that mechanical interactions between the extracellular matrix (ECM) and cell surface receptors as well as physical interactions between neighboring cells play important roles in regulating the signaling pathways controlling the developmental processes of the heart. Interdisciplinary efforts have made it apparent that the influence of the ECM on cellular behavior occurs through a multitude of physical mechanisms, such as ECM boundary conditions, elasticity, and the propagation of mechanical signals to intracellular compartments, such as the nucleus. In addition to experimental studies, a number of mathematical models have been developed that attempt to capture the interplay between cells and their local microenvironment and the influence these interactions have on cellular self-assembly and functional behavior. Nevertheless, many questions remain unanswered concerning the mechanism through which physical interactions between cardiomyocytes and their environment are translated into biochemical cellular responses and how these signaling modalities can be utilized in vitro to fabricate myocardial tissue constructs from stem cell-derived cardiomyocytes that more faithfully represent their in vivo counterpart. These studies represent a broad effort to characterize biological form as a conduit for information transfer that spans the nanometer length scale of proteins to the meter length scale of the patient and may yield new insights into the contribution of mechanotransduction into heart development and disease.
Figures

References
-
- Alenghat FJ, Ingber DE, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;2002(119):pe6. - PubMed
-
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RDG. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–826. - PubMed
-
- Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L1–L8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

