Functional characterization of an active Rag-like transposase
- PMID: 22773102
- PMCID: PMC3414642
- DOI: 10.1038/nsmb.2338
Functional characterization of an active Rag-like transposase
Abstract
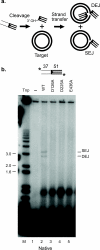
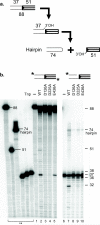
The formation of diverse immunoglobulin genes results in part from Rag protein-mediated DNA double-strand breaks at the edges of immunoglobulin gene segments, followed by combinatorial reassembly of these segments. We report that a Transib transposase from the insect Helicoverpa zea is active in vitro and that its breakage and joining activities mimic those of Rag, providing strong evidence that Rag and Transib transposases were derived from a common progenitor.
Figures

Similar articles
-
New insights into the evolutionary origins of the recombination-activating gene proteins and V(D)J recombination.FEBS J. 2017 Jun;284(11):1590-1605. doi: 10.1111/febs.13990. Epub 2017 Jan 6. FEBS J. 2017. PMID: 27973733 Free PMC article. Review.
-
Structures of a RAG-like transposase during cut-and-paste transposition.Nature. 2019 Nov;575(7783):540-544. doi: 10.1038/s41586-019-1753-7. Epub 2019 Nov 13. Nature. 2019. PMID: 31723264 Free PMC article.
-
Transposition of Mutator-like transposable elements (MULEs) resembles hAT and Transib elements and V(D)J recombination.Nucleic Acids Res. 2017 Jun 20;45(11):6644-6655. doi: 10.1093/nar/gkx357. Nucleic Acids Res. 2017. PMID: 28482040 Free PMC article.
-
RAG1 core and V(D)J recombination signal sequences were derived from Transib transposons.PLoS Biol. 2005 Jun;3(6):e181. doi: 10.1371/journal.pbio.0030181. Epub 2005 May 24. PLoS Biol. 2005. PMID: 15898832 Free PMC article.
-
The origins of the Rag genes--from transposition to V(D)J recombination.Semin Immunol. 2010 Feb;22(1):10-6. doi: 10.1016/j.smim.2009.11.004. Epub 2009 Dec 9. Semin Immunol. 2010. PMID: 20004590 Free PMC article. Review.
Cited by
-
Biochemical Characterization of Kat1: a Domesticated hAT-Transposase that Induces DNA Hairpin Formation and MAT-Switching.Sci Rep. 2016 Feb 23;6:21671. doi: 10.1038/srep21671. Sci Rep. 2016. PMID: 26902909 Free PMC article.
-
New insights into the evolutionary origins of the recombination-activating gene proteins and V(D)J recombination.FEBS J. 2017 Jun;284(11):1590-1605. doi: 10.1111/febs.13990. Epub 2017 Jan 6. FEBS J. 2017. PMID: 27973733 Free PMC article. Review.
-
Structures of a RAG-like transposase during cut-and-paste transposition.Nature. 2019 Nov;575(7783):540-544. doi: 10.1038/s41586-019-1753-7. Epub 2019 Nov 13. Nature. 2019. PMID: 31723264 Free PMC article.
-
Structural insights into the mechanism of double strand break formation by Hermes, a hAT family eukaryotic DNA transposase.Nucleic Acids Res. 2018 Nov 2;46(19):10286-10301. doi: 10.1093/nar/gky838. Nucleic Acids Res. 2018. PMID: 30239795 Free PMC article.
-
The SpTransformer Gene Family (Formerly Sp185/333) in the Purple Sea Urchin and the Functional Diversity of the Anti-Pathogen rSpTransformer-E1 Protein.Front Immunol. 2017 Jun 30;8:725. doi: 10.3389/fimmu.2017.00725. eCollection 2017. Front Immunol. 2017. PMID: 28713368 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

