The p97/VCP ATPase is critical in muscle atrophy and the accelerated degradation of muscle proteins
- PMID: 22773186
- PMCID: PMC3411080
- DOI: 10.1038/emboj.2012.178
The p97/VCP ATPase is critical in muscle atrophy and the accelerated degradation of muscle proteins
Abstract
The p97/VCP ATPase complex facilitates the extraction and degradation of ubiquitinated proteins from larger structures. We therefore studied if p97 participates to the rapid degradation of myofibrillar proteins during muscle atrophy. Electroporation of a dominant negative p97 (DNp97), but not the WT, into mouse muscle reduced fibre atrophy caused by denervation and food deprivation. DNp97 (acting as a substrate-trap) became associated with specific myofibrillar proteins and its cofactors, Ufd1 and p47, and caused accumulation of ubiquitinated components of thin and thick filaments, which suggests a role for p97 in extracting ubiquitinated proteins from myofibrils. DNp97 expression in myotubes reduced overall proteolysis by proteasomes and lysosomes and blocked the accelerated proteolysis induced by FoxO3, which is essential for atrophy. Expression of p97, Ufd1 and p47 increases following denervation, at times when myofibrils are rapidly degraded. Surprisingly, p97 inhibition, though toxic to most cells, caused rapid growth of myotubes (without enhancing protein synthesis) and hypertrophy of adult muscles. Thus, p97 restrains post-natal muscle growth, and during atrophy, is essential for the accelerated degradation of most muscle proteins.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



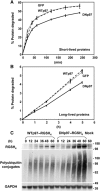


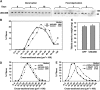
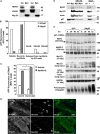
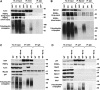

References
-
- Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ (1997) Wasting as independent risk factor for mortality in chronic heart failure. Lancet 349: 1050–1053 - PubMed
-
- Beskow A, Grimberg KB, Bott LC, Salomons FA, Dantuma NP, Young P (2009) A conserved unfoldase activity for the p97 AAA-ATPase in proteasomal degradation. J Mol Biol 394: 732–746 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

