Acidification of the synaptic cleft of cone photoreceptor terminal controls the amount of transmitter release, thereby forming the receptive field surround in the vertebrate retina
- PMID: 22773408
- PMCID: PMC10717482
- DOI: 10.1007/s12576-012-0220-0
Acidification of the synaptic cleft of cone photoreceptor terminal controls the amount of transmitter release, thereby forming the receptive field surround in the vertebrate retina
Abstract
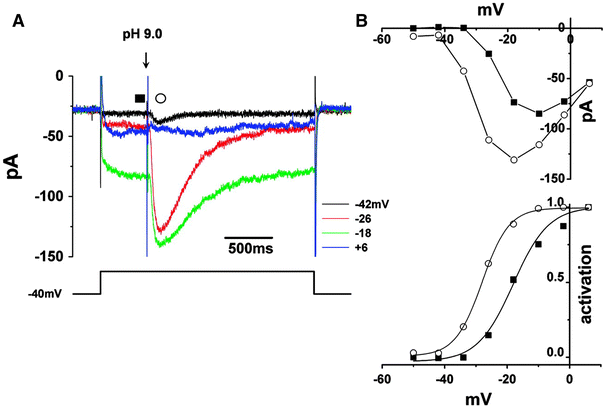
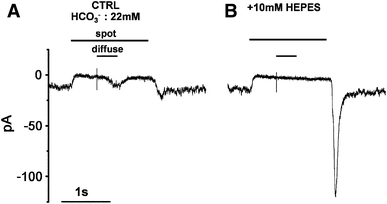
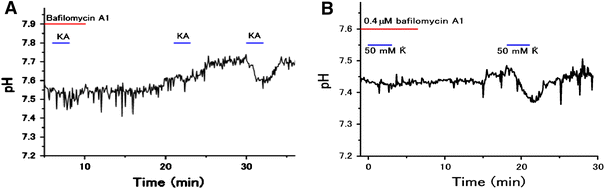
In the vertebrate retina, feedback from horizontal cells (HCs) to cone photoreceptors plays a key role in the formation of the center-surround receptive field of retinal cells, which induces contrast enhancement of visual images. The mechanism underlying surround inhibition is not fully understood. In this review, we discuss this issue, focusing on our recent hypothesis that acidification of the synaptic cleft of the cone photoreceptor terminal causes this inhibition by modulating the Ca channel of the terminals. We present evidence that the acidification is caused by proton excretion from HCs by a vacuolar type H(+) pump. Recent publications supporting or opposing our hypothesis are discussed.
Figures







Similar articles
-
pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels.J Gen Physiol. 2003 Dec;122(6):657-71. doi: 10.1085/jgp.200308863. Epub 2003 Nov 10. J Gen Physiol. 2003. PMID: 14610018 Free PMC article.
-
Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse.J Neurosci. 2005 Apr 20;25(16):4108-17. doi: 10.1523/JNEUROSCI.5253-04.2005. J Neurosci. 2005. PMID: 15843613 Free PMC article.
-
Sources of protons and a role for bicarbonate in inhibitory feedback from horizontal cells to cones in Ambystoma tigrinum retina.J Physiol. 2016 Nov 15;594(22):6661-6677. doi: 10.1113/JP272533. Epub 2016 Jul 21. J Physiol. 2016. PMID: 27345444 Free PMC article.
-
The feedback pathway from horizontal cells to cones. A mini review with a look ahead.Vision Res. 1999 Jul;39(15):2449-68. doi: 10.1016/s0042-6989(99)00043-7. Vision Res. 1999. PMID: 10396615 Review.
-
Horizontal Cell Feedback to Cone Photoreceptors in Mammalian Retina: Novel Insights From the GABA-pH Hybrid Model.Front Cell Neurosci. 2020 Nov 4;14:595064. doi: 10.3389/fncel.2020.595064. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33328894 Free PMC article. Review.
Cited by
-
Versatile functional roles of horizontal cells in the retinal circuit.Sci Rep. 2017 Jul 17;7(1):5540. doi: 10.1038/s41598-017-05543-2. Sci Rep. 2017. PMID: 28717219 Free PMC article.
-
Voltage-Gated Calcium Channels: Key Players in Sensory Coding in the Retina and the Inner Ear.Physiol Rev. 2018 Oct 1;98(4):2063-2096. doi: 10.1152/physrev.00030.2017. Physiol Rev. 2018. PMID: 30067155 Free PMC article. Review.
-
Specialized synaptic pathway for chromatic signals beneath S-cone photoreceptors is common to human, Old and New World primates.J Opt Soc Am A Opt Image Sci Vis. 2014 Apr 1;31(4):A189-94. doi: 10.1364/JOSAA.31.00A189. J Opt Soc Am A Opt Image Sci Vis. 2014. PMID: 24695169 Free PMC article.
-
Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina.Nat Neurosci. 2014 Feb;17(2):262-8. doi: 10.1038/nn.3627. Epub 2014 Jan 19. Nat Neurosci. 2014. PMID: 24441679 Free PMC article.
-
Activation of retinal glial (Müller) cells by extracellular ATP induces pronounced increases in extracellular H+ flux.PLoS One. 2018 Feb 21;13(2):e0190893. doi: 10.1371/journal.pone.0190893. eCollection 2018. PLoS One. 2018. PMID: 29466379 Free PMC article.
References
-
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

