Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality
- PMID: 22773591
- PMCID: PMC3430948
- DOI: 10.2215/CJN.01100112
Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality
Abstract
Background: Acidosis and transplantation are associated with increased risk of bone disturbances. This study aimed to assess bone morphology and metabolism in acidotic patients with a renal graft, and to ameliorate bone characteristics by restoration of acid/base homeostasis with potassium citrate.
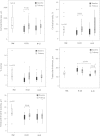
Methods: This was a 12-month controlled, randomized, interventional trial that included 30 renal transplant patients with metabolic acidosis (S-[HCO(3)(-)] <24 mmol/L) undergoing treatment with either potassium citrate to maintain S-[HCO(3)(-)] >24 mmol/L, or potassium chloride (control group). Iliac crest bone biopsies and dual-energy X-ray absorptiometry were performed at baseline and after 12 months of treatment. Bone biopsies were analyzed by in vitro micro-computed tomography and histomorphometry, including tetracycline double labeling. Serum biomarkers of bone turnover were measured at baseline and study end. Twenty-three healthy participants with normal kidney function comprised the reference group.
Results: Administration of potassium citrate resulted in persisting normalization of S-[HCO(3)(-)] versus potassium chloride. At 12 months, bone surface, connectivity density, cortical thickness, and cortical porosity were better preserved with potassium citrate than with potassium chloride, respectively. Serological biomarkers and bone tetracycline labeling indicate higher bone turnover with potassium citrate versus potassium chloride. In contrast, no relevant changes in bone mineral density were detected by dual-energy X-ray absorptiometry.
Conclusions: Treatment with potassium citrate in renal transplant patients is efficient and well tolerated for correction of metabolic acidosis and may be associated with improvement in bone quality. This study is limited by the heterogeneity of the investigated population with regard to age, sex, and transplant vintage.
Trial registration: ClinicalTrials.gov NCT00913796.
Figures





References
-
- Chiu MY, Sprague SM, Bruce DS, Woodle ES, Thistlethwaite JR, Jr, Josephson MA: Analysis of fracture prevalence in kidney-pancreas allograft recipients. J Am Soc Nephrol 9: 677–683, 1998 - PubMed
-
- Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, Saiphoo C, Fenton SS, Segre GV: The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int 43: 436–442, 1993 - PubMed
-
- Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH: High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol 11: 1093–1099, 2000 - PubMed
-
- Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, Tellis V, Greenstein S, Schechner R, Figueroa K, McDonough P, Wang G, Malluche H: Prevention of bone loss in renal transplant recipients: A prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 14: 2669–2676, 2003 - PubMed
-
- Movsowitz C, Schlosberg M, Epstein S, Ismail F, Fallon M, Thomas S: Combined treatment with cyclosporin A and cortisone acetate minimizes the adverse bone effects of either agent alone. J Orthop Res 8: 635–641, 1990 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

