Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry
- PMID: 22773654
- PMCID: PMC3426709
- DOI: 10.1128/AEM.01257-12
Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry
Abstract
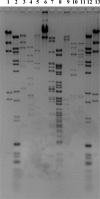
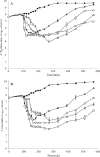
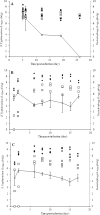
Salmonella remains the major cause of food-borne diseases worldwide, with chickens known to be the main reservoir for this zoonotic pathogen. Among the many approaches to reducing Salmonella colonization of broilers, bacteriophage offers several advantages. In this study, three bacteriophages (UAB_Phi20, UAB_Phi78, and UAB_Phi87) obtained from our collection that exhibited a broad host range against Salmonella enterica serovar Enteritidis and Salmonella enterica serovar Typhimurium were characterized with respect to morphology, genome size, and restriction patterns. A cocktail composed of the three bacteriophages was more effective in promoting the lysis of S. Enteritidis and S. Typhimurium cultures than any of the three bacteriophages alone. In addition, the cocktail was able to lyse the Salmonella enterica serovars Virchow, Hadar, and Infantis. The effectiveness of the bacteriophage cocktail in reducing the concentration of S. Typhimurium was tested in two animal models using different treatment schedules. In the mouse model, 50% survival was obtained when the cocktail was administered simultaneously with bacterial infection and again at 6, 24, and 30 h postinfection. Likewise, in the White Leghorn chicken specific-pathogen-free (SPF) model, the best results, defined as a reduction of Salmonella concentration in the chicken cecum, were obtained when the bacteriophage cocktail was administered 1 day before or just after bacterial infection and then again on different days postinfection. Our results show that frequent treatment of the chickens with bacteriophage, and especially prior to colonization of the intestinal tract by Salmonella, is required to achieve effective bacterial reduction over time.
Figures



References
-
- Andreatti Filho RL, et al. 2007. Ability of bacteriophages isolated from different sources to reduce Salmonella enterica serovar Enteritidis in vitro and in vivo. Poult. Sci. 86:1904–1909 - PubMed
-
- Ausubel FM, et al. 1987. Current protocols in molecular biology, vol 1 Greene Publishing/Wiley-Interscience, New York, NY
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

