Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation
- PMID: 22773696
- PMCID: PMC3774493
- DOI: 10.1152/ajplung.00408.2011
Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation
Abstract
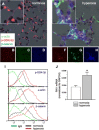
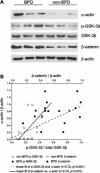
In bronchopulmonary dysplasia (BPD), alveolar septa are thickened with collagen and α-smooth muscle actin-, transforming growth factor (TGF)-β-positive myofibroblasts. We examined the biochemical mechanisms underlying myofibroblastic differentiation, focusing on the role of glycogen synthase kinase-3β (GSK-3β)/β-catenin signaling pathway. In the cytoplasm, β-catenin is phosphorylated on the NH(2) terminus by constitutively active GSK-3β, favoring its degradation. Upon TGF-β stimulation, GSK-3β is phosphorylated and inactivated, allowing β-catenin to translocate to the nucleus, where it activates transcription of genes involved in myofibroblastic differentiation. We examined the role of β-catenin in TGF-β1-induced myofibroblastic differentiation of neonatal lung mesenchymal stromal cells (MSCs) isolated from tracheal aspirates of premature infants with respiratory distress. TGF-β1 increased β-catenin expression and nuclear translocation. Transduction of cells with GSK-3β S9A, a nonphosphorylatable, constitutively active mutant that favors β-catenin degradation, blocked TGF-β1-induced myofibroblastic differentiation. Furthermore, transduction of MSCs with ΔN-catenin, a truncation mutant that cannot be phosphorylated on the NH(2) terminus by GSK-3β and is not degraded, was sufficient for myofibroblastic differentiation. In vivo, hyperoxic exposure of neonatal mice increases expression of β-catenin in α-smooth muscle actin-positive myofibroblasts. Similar changes were found in lungs of infants with BPD. Finally, low-passage unstimulated MSCs from infants developing BPD showed higher phospho-GSK-3β, β-catenin, and α-actin content compared with MSCs from infants not developing this disease, and phospho-GSK-3β and β-catenin each correlated with α-actin content. We conclude that phospho-GSK-3β/β-catenin signaling regulates α-smooth muscle actin expression, a marker of myofibroblast differentiation, in vitro and in vivo. This pathway appears to be activated in lung mesenchymal cells from patients with BPD.
Figures






References
-
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994. - PubMed
-
- Alapati D, Rong M, Chen S, Hehre D, Rodriguez MM, Lipson KE, Wu S. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol 45: 1169–1177, 2011. - PubMed
-
- Armstrong DD, Esser KA. Wnt/β-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol 289: C853–C859, 2005. - PubMed
-
- Ben-Ze'ev A, Shtutman M, Zhurinsky J. The integration of cell adhesion with gene expression: The role of β-catenin. Exp Cell Res 261: 75–82, 2000. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

