Accelerated renal disease is associated with the development of metabolic syndrome in a glucolipotoxic mouse model
- PMID: 22773754
- PMCID: PMC3424461
- DOI: 10.1242/dmm.009266
Accelerated renal disease is associated with the development of metabolic syndrome in a glucolipotoxic mouse model
Abstract
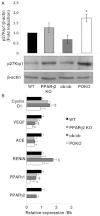
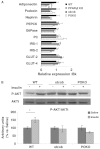
Individuals with metabolic syndrome are at high risk of developing chronic kidney disease (CKD) through unclear pathogenic mechanisms. Obesity and diabetes are known to induce glucolipotoxic effects in metabolically relevant organs. However, the pathogenic role of glucolipotoxicity in the aetiology of diabetic nephropathy is debated. We generated a murine model, the POKO mouse, obtained by crossing the peroxisome proliferator-activated receptor gamma 2 (PPARγ2) knockout (KO) mouse into a genetically obese ob/ob background. We have previously shown that the POKO mice showed: hyperphagia, insulin resistance, hyperglycaemia and dyslipidaemia as early as 4 weeks of age, and developed a complete loss of normal β-cell function by 16 weeks of age. Metabolic phenotyping of the POKO model has led to investigation of the structural and functional changes in the kidney and changes in blood pressure in these mice. Here we demonstrate that the POKO mouse is a model of renal disease that is accelerated by high levels of glucose and lipid accumulation. Similar to ob/ob mice, at 4 weeks of age these animals exhibited an increased urinary albumin:creatinine ratio and significantly increased blood pressure, but in contrast showed a significant increase in the renal hypertrophy index and an associated increase in p27(Kip1) expression compared with their obese littermates. Moreover, at 4 weeks of age POKO mice showed insulin resistance, an alteration of lipid metabolism and glomeruli damage associated with increased transforming growth factor beta (TGFβ) and parathyroid hormone-related protein (PTHrP) expression. At this age, levels of proinflammatory molecules, such as monocyte chemoattractant protein-1 (MCP-1), and fibrotic factors were also increased at the glomerular level compared with levels in ob/ob mice. At 12 weeks of age, renal damage was fully established. These data suggest an accelerated lesion through glucolipotoxic effects in the renal pathogenesis in POKO mice.
Figures





References
-
- Becker B., Kronenberg F., Kielstein J. T., Haller H., Morath C., Ritz E., Fliser D. (2005). Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J. Am. Soc. Nephrol. 16, 1091–1098 - PubMed
-
- Bianchi S., Bigazzi R., Caiazza A., Campese V. M. (2003). A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease. Am. J. Kidney Dis. 41, 565–570 - PubMed
-
- Chavez J. A., Holland W. L., Bar J., Sandhoff K., Summers S. A. (2005). Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 280, 20148–20153 - PubMed
-
- Chou E., Suzuma I., Way K. J., Opland D., Clermont A. C., Naruse K., Suzuma K., Bowling N. L., Vlahos C. J., Aiello L. P., et al. (2002). Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 105, 373–379 - PubMed
-
- Chung B. H., Li C., Sun B. K., Lim S. W., Ahn K. O., Yang J. H., Choi Y. H., Yoon K. H., Sugawara A., Ito S., et al. (2005). Rosiglitazone protects against cyclosporine-induced pancreatic and renal injury in rats. Am. J. Transplant. 5, 1856–1867 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

