Impact of hydroxyl radical-induced injury on calcium handling and myofilament sensitivity in isolated myocardium
- PMID: 22773772
- PMCID: PMC3472469
- DOI: 10.1152/japplphysiol.01424.2011
Impact of hydroxyl radical-induced injury on calcium handling and myofilament sensitivity in isolated myocardium
Abstract
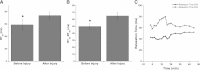
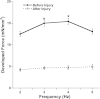
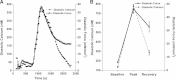
Hydroxyl radicals (OH) are involved in the pathogenesis of reperfusion injury and are observed in acute heart failure, stroke, and myocardial infarction. Two different subcellular defects are involved in the pathogenesis of OH injury, deranged calcium handling, and alterations of myofilament responsiveness, but their temporal impact on contractile function is not resolved. Initially, after brief OH exposure, there is a corresponding marked increase in diastolic calcium and diastolic force. We followed these parameters until a new steady-state level was reached at ~45 min post-OH exposure. At this new baseline, diastolic calcium had returned to near-normal, pre-OH levels, whereas diastolic force remained markedly elevated. An increased calcium sensitivity was observed at the new baseline after OH-induced injury compared with the pre-OH state. The acute injury that occurs after OH exposure is mainly due to calcium overload, while the later sustained myocardial dysfunction is mainly due to the altered/increased myofilament responsiveness.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

