Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation
- PMID: 22773805
- PMCID: PMC3409786
- DOI: 10.1073/pnas.1118425109
Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation
Abstract
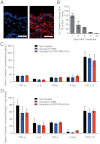
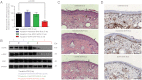
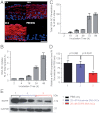
Topical application of nucleic acids offers many potential therapeutic advantages for suppressing genes in the skin, and potentially for systemic gene delivery. However, the epidermal barrier typically precludes entry of gene-suppressing therapy unless the barrier is disrupted. We now show that spherical nucleic acid nanoparticle conjugates (SNA-NCs), gold cores surrounded by a dense shell of highly oriented, covalently immobilized siRNA, freely penetrate almost 100% of keratinocytes in vitro, mouse skin, and human epidermis within hours after application. Significantly, these structures can be delivered in a commercial moisturizer or phosphate-buffered saline, and do not require barrier disruption or transfection agents, such as liposomes, peptides, or viruses. SNA-NCs targeting epidermal growth factor receptor (EGFR), an important gene for epidermal homeostasis, are > 100-fold more potent and suppress longer than siRNA delivered with commercial lipid agents in cultured keratinocytes. Topical delivery of 1.5 uM EGFR siRNA (50 nM SNA-NCs) for 3 wk to hairless mouse skin almost completely abolishes EGFR expression, suppresses downstream ERK phosphorylation, and reduces epidermal thickness by almost 40%. Similarly, EGFR mRNA in human skin equivalents is reduced by 52% after 60 h of treatment with 25 nM EGFR SNA-NCs. Treated skin shows no clinical or histological evidence of toxicity. No cytokine activation in mouse blood or tissue samples is observed, and after 3 wk of topical skin treatment, the SNA structures are virtually undetectable in internal organs. SNA conjugates may be promising agents for personalized, topically delivered gene therapy of cutaneous tumors, skin inflammation, and dominant negative genetic skin disorders.
Conflict of interest statement
Conflict of interest statement: This technology has been licensed from Northwestern University by AuraSense Therapeutics, LLC. C.A.M., A.S.P., and D.A.G. have financial interests in AuraSense Therapeutics, LLC.
Figures

Similar articles
-
Using siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation in psoriasis.J Control Release. 2017 Dec 28;268:259-268. doi: 10.1016/j.jconrel.2017.10.034. Epub 2017 Oct 23. J Control Release. 2017. PMID: 29074408
-
siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown.Proc Natl Acad Sci U S A. 2015 May 5;112(18):5573-8. doi: 10.1073/pnas.1505951112. Epub 2015 Apr 20. Proc Natl Acad Sci U S A. 2015. PMID: 25902507 Free PMC article.
-
Epidermal SR-A Complexes Are Lipid Raft Based and Promote Nucleic Acid Nanoparticle Uptake.J Invest Dermatol. 2021 Jun;141(6):1428-1437.e8. doi: 10.1016/j.jid.2020.10.027. Epub 2020 Dec 30. J Invest Dermatol. 2021. PMID: 33385397 Free PMC article.
-
Therapeutic applications of spherical nucleic acids.Cancer Treat Res. 2015;166:23-50. doi: 10.1007/978-3-319-16555-4_2. Cancer Treat Res. 2015. PMID: 25895863 Review.
-
Recent advances in the epidermal growth factor receptor/ligand system biology on skin homeostasis and keratinocyte stem cell regulation.J Dermatol Sci. 2013 Nov;72(2):81-6. doi: 10.1016/j.jdermsci.2013.05.009. Epub 2013 Jun 13. J Dermatol Sci. 2013. PMID: 23819985 Review.
Cited by
-
A Simple Colorimetric Assay of Bleomycin-Mediated DNA Cleavage Utilizing Double-Stranded DNA-Modified Gold Nanoparticles.Chembiochem. 2023 Jan 3;24(1):e202200451. doi: 10.1002/cbic.202200451. Epub 2022 Oct 25. Chembiochem. 2023. PMID: 36156837 Free PMC article.
-
The Limitless Future of RNA Therapeutics.Front Bioeng Biotechnol. 2021 Mar 18;9:628137. doi: 10.3389/fbioe.2021.628137. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33816449 Free PMC article. Review.
-
Pigmy MicroRNA: surveillance cops in Therapies kingdom.Mol Med. 2016 Dec;22:759-775. doi: 10.2119/molmed.2016.00136. Epub 2016 Sep 28. Mol Med. 2016. PMID: 27704139 Free PMC article.
-
Spherical Nucleic Acids as Precision Therapeutics for the Treatment of Cancer-From Bench to Bedside.Cancers (Basel). 2022 Mar 23;14(7):1615. doi: 10.3390/cancers14071615. Cancers (Basel). 2022. PMID: 35406387 Free PMC article. Review.
-
Spherical nucleic acids: a whole new ball game.Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13231-3. doi: 10.1073/pnas.1313483110. Proc Natl Acad Sci U S A. 2013. PMID: 23943910 Free PMC article. No abstract available.
References
-
- Von Hoff DD, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. New Engl J Med. 2009;361:1164–1172. - PubMed
-
- Griffiths CEM, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. New Engl J Med. 2010;362:118–128. - PubMed
-
- Mountzios G, Syrigos KN. A benefit-risk assessment of erlotinib in non-small-cell lung cancer and pancreatic cancer. Drug Safety. 2011;34:175–186. - PubMed
-
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

