Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile
- PMID: 22773807
- PMCID: PMC3409760
- DOI: 10.1073/pnas.1207670109
Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile
Abstract
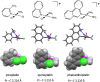
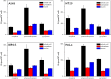
Monofunctional platinum(II) complexes of general formula cis-[Pt(NH(3))(2)(N-heterocycle)Cl]Cl bind DNA at a single site, inducing little distortion in the double helix. Despite this behavior, these compounds display significant antitumor properties, with a different spectrum of activity than that of classic bifunctional cross-linking agents like cisplatin. To discover the most potent monofunctional platinum(II) compounds, the N-heterocycle was systematically varied to generate a small library of new compounds, with guidance from the X-ray structure of RNA polymerase II (Pol II) stalled at a monofunctional pyriplatin-DNA adduct. In pyriplatin, the N-heterocycle is pyridine. The most effective complex evaluated was phenanthriplatin, cis-[Pt(NH(3))(2)(phenanthridine)Cl]NO(3), which exhibits significantly greater activity than the Food and Drug Administration-approved drugs cisplatin and oxaliplatin. Studies of phenanthriplatin in the National Cancer Institute 60-cell tumor panel screen revealed a spectrum of activity distinct from that of these clinically validated anticancer agents. The cellular uptake of phenanthriplatin is substantially greater than that of cisplatin and pyriplatin because of the hydrophobicity of the phenanthridine ligand. Phenanthriplatin binds more effectively to 5'-deoxyguanosine monophosphate than to N-acetyl methionine, whereas pyriplatin reacts equally well with both reagents. This chemistry supports DNA as a viable cellular target for phenanthriplatin and suggests that it may avoid cytoplasmic platinum scavengers with sulfur-donor ligands that convey drug resistance. With the use of globally platinated Gaussia luciferase vectors, we determined that phenanthriplatin inhibits transcription in live mammalian cells as effectively as cisplatin, despite its inability to form DNA cross-links.
Conflict of interest statement
Conflict of interest statement: S.J.L. has financial interest in Blend Therapeutics.
Figures
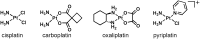
Similar articles
-
Anti-cancer characteristics and ototoxicity of platinum(II) amine complexes with only one leaving ligand.PLoS One. 2018 Mar 7;13(3):e0192505. doi: 10.1371/journal.pone.0192505. eCollection 2018. PLoS One. 2018. PMID: 29513752 Free PMC article.
-
Structural and mechanistic studies of polymerase η bypass of phenanthriplatin DNA damage.Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9133-8. doi: 10.1073/pnas.1405739111. Epub 2014 Jun 9. Proc Natl Acad Sci U S A. 2014. PMID: 24927576 Free PMC article.
-
Effect of a monofunctional phenanthriplatin-DNA adduct on RNA polymerase II transcriptional fidelity and translesion synthesis.J Am Chem Soc. 2013 Sep 4;135(35):13054-61. doi: 10.1021/ja405475y. Epub 2013 Aug 25. J Am Chem Soc. 2013. PMID: 23927577 Free PMC article.
-
Understanding and improving platinum anticancer drugs--phenanthriplatin.Anticancer Res. 2014 Jan;34(1):471-6. Anticancer Res. 2014. PMID: 24403503 Free PMC article. Review.
-
Fresh platinum complexes with promising antitumor activity.Anticancer Agents Med Chem. 2010 Jun;10(5):396-411. doi: 10.2174/1871520611009050396. Anticancer Agents Med Chem. 2010. PMID: 20545618 Review.
Cited by
-
Chromatin folding and DNA replication inhibition mediated by a highly antitumor-active tetrazolato-bridged dinuclear platinum(II) complex.Sci Rep. 2016 Apr 20;6:24712. doi: 10.1038/srep24712. Sci Rep. 2016. PMID: 27094881 Free PMC article.
-
Investigations of the binding of [Pt2(DTBPA)Cl2](II) and [Pt2(TPXA)Cl2](II) to DNA via various cross-linking modes.Int J Mol Sci. 2013 Sep 26;14(10):19556-86. doi: 10.3390/ijms141019556. Int J Mol Sci. 2013. PMID: 24077126 Free PMC article.
-
Effect of lanthanide complex structure on cell viability and association.Inorg Chem. 2014 Jun 16;53(12):6013-21. doi: 10.1021/ic500282n. Epub 2014 Jun 5. Inorg Chem. 2014. PMID: 24901440 Free PMC article.
-
Redesigning the DNA-targeted chromophore in platinum-acridine anticancer agents: a structure-activity relationship study.Chemistry. 2014 Dec 1;20(49):16174-87. doi: 10.1002/chem.201404845. Epub 2014 Oct 10. Chemistry. 2014. PMID: 25302716 Free PMC article.
-
Reimagining Pt(II) Anticancer Agents: The Role of Ferrocene in Monofunctional Chemotherapeutic Compounds.Inorg Chem. 2025 Jun 16;64(23):11497-11509. doi: 10.1021/acs.inorgchem.5c00704. Epub 2025 Jun 2. Inorg Chem. 2025. PMID: 40455123 Free PMC article.
References
-
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. - PubMed
-
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999;99:2467–2498. - PubMed
-
- Jung YW, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem Rev. 2007;107:1387–1407. - PubMed
-
- Hollis LS, Amundsen AR, Stern EW. Chemical and biological properties of a new series of cis-diammineplatinum(II) antitumor agents containing three nitrogen donors: cis-[Pt(NH3)2(N-donor)Cl]+ J Med Chem. 1989;32:128–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

