The Wnt receptor Ryk plays a role in mammalian planar cell polarity signaling
- PMID: 22773843
- PMCID: PMC3436188
- DOI: 10.1074/jbc.M112.362681
The Wnt receptor Ryk plays a role in mammalian planar cell polarity signaling
Abstract
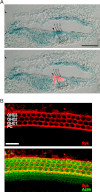
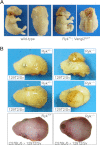
Wnts are essential for a wide range of developmental processes, including cell growth, division, and differentiation. Some of these processes signal via the planar cell polarity (PCP) pathway, which is a β-catenin-independent Wnt signaling pathway. Previous studies have shown that Ryk, a member of the receptor tyrosine kinase family, can bind to Wnts. Ryk is required for normal axon guidance and neuronal differentiation during development. Here, we demonstrate that mammalian Ryk interacts with the Wnt/PCP pathway. In vitro analysis showed that the Wnt inhibitory factor domain of Ryk was necessary for Wnt binding. Detailed analysis of two vertebrate model organisms showed Ryk phenotypes consistent with PCP signaling. In zebrafish, gene knockdown using morpholinos revealed a genetic interaction between Ryk and Wnt11 during the PCP pathway-regulated process of embryo convergent extension. Ryk-deficient mouse embryos displayed disrupted polarity of stereociliary hair cells in the cochlea, a characteristic of disturbed PCP signaling. This PCP defect was also observed in mouse embryos that were double heterozygotes for Ryk and Looptail (containing a mutation in the core Wnt/PCP pathway gene Vangl2) but not in either of the single heterozygotes, suggesting a genetic interaction between Ryk and Vangl2. Co-immunoprecipitation studies demonstrated that RYK and VANGL2 proteins form a complex, whereas RYK also activated RhoA, a downstream effector of PCP signaling. Overall, our data suggest an important role for Ryk in Wnt/planar cell polarity signaling during vertebrate development via the Vangl2 signaling pathway, as demonstrated in the mouse cochlea.
Figures






References
-
- Seifert J. R., Mlodzik M. (2007) Frizzled/PCP signaling. A conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126–138 - PubMed
-
- Keller R. (2002) Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298, 1950–1954 - PubMed
-
- Tada M., Smith J. C. (2000) Xwnt11 is a target of Xenopus Brachyury; Regulation of gastrulation movements via Dishevelled but not through the canonical Wnt pathway. Development 127, 2227–2238 - PubMed
-
- Wallingford J. B., Rowning B. A., Vogeli K. M., Rothbächer U., Fraser S. E., Harland R. M. (2000) Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405, 81–85 - PubMed
-
- Heisenberg C. P., Tada M., Rauch G. J., Saúde L., Concha M. L., Geisler R., Stemple D. L., Smith J. C., Wilson S. W. (2000) Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

