Mutant NADH dehydrogenase subunit 4 gene delivery to mitochondria by targeting sequence-modified adeno-associated virus induces visual loss and optic atrophy in mice
- PMID: 22773905
- PMCID: PMC3388991
Mutant NADH dehydrogenase subunit 4 gene delivery to mitochondria by targeting sequence-modified adeno-associated virus induces visual loss and optic atrophy in mice
Abstract
Purpose: Although mutated G11778A NADH ubiquinone oxidoreductase subunit 4 (ND4) mitochondrial DNA (mtDNA) is firmly linked to the blindness of Leber hereditary optic neuropathy (LHON), a bona fide animal model system with mutated mtDNA complex I subunits that would enable probing the pathogenesis of optic neuropathy and testing potential avenues for therapy has yet to be developed.
Methods: The mutant human ND4 gene with a guanine to adenine transition at position 11778 with an attached FLAG epitope under control of the mitochondrial heavy strand promoter (HSP) was inserted into a modified self-complementary (sc) adeno-associated virus (AAV) backbone. The HSP-ND4FLAG was directed toward the mitochondria by adding the 23 amino acid cytochrome oxidase subunit 8 (COX8) presequence fused in frame to the N-terminus of green fluorescent protein (GFP) into the AAV2 capsid open reading frame. The packaged scAAV-HSP mutant ND4 was injected into the vitreous cavity of normal mice (OD). Contralateral eyes received scAAV-GFP (OS). Translocation and integration of mutant human ND4 in mouse mitochondria were assessed with PCR, reverse transcription-polymerase chain reaction (RT-PCR), sequencing, immunoblotting, and immunohistochemistry. Visual function was monitored with serial pattern electroretinography (PERG) and in vivo structure with spectral domain optical coherence tomography (OCT). Animals were euthanized at 1 year and processed for light and transmission electron microscopy.
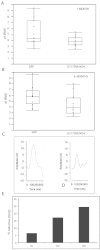
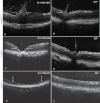
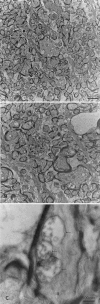
Results: The PCR products of the mitochondrial and nuclear DNA extracted from infected retinas and optic nerves gave the expected 500 base pair bands. RT-PCR confirmed transcription of the mutant human ND4 DNA in mice. DNA sequencing confirmed that the PCR and RT-PCR products were mutant human ND4 (OD only). Immunoblotting revealed the expression of mutant ND4FLAG (OD only). Pattern electroretinograms showed a significant decrement in retinal ganglion cell function OD relative to OS at 1 month and 6 months after AAV injections. Spectral domain optical coherence tomography showed optic disc edema starting at 1 month post injection followed by optic nerve head atrophy with marked thinning of the inner retina at 1 year. Histopathology of optic nerve cross sections revealed reductions in the optic nerve diameters of OD versus OS where transmission electron microscopy revealed significant loss of optic nerve axons in mutant ND4 injected eyes where some remaining axons were still in various stages of irreversible degeneration with electron dense aggregation. Electron lucent mitochondria accumulated in swollen axons where fusion of mitochondria was also evident.
Conclusions: Due to the UGA codon at amino acid 16, mutant G11778A ND4 was translated only in the mitochondria where its expression led to significant loss of visual function, loss of retinal ganglion cells, and optic nerve degeneration recapitulating the hallmarks of human LHON.
Figures






Similar articles
-
Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial.JAMA Ophthalmol. 2014 Apr 1;132(4):409-20. doi: 10.1001/jamaophthalmol.2013.7630. JAMA Ophthalmol. 2014. PMID: 24457989 Free PMC article. Clinical Trial.
-
Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system.Invest Ophthalmol Vis Sci. 2009 Sep;50(9):4205-14. doi: 10.1167/iovs.08-3214. Epub 2009 Apr 22. Invest Ophthalmol Vis Sci. 2009. PMID: 19387075 Free PMC article.
-
LHON gene therapy vector prevents visual loss and optic neuropathy induced by G11778A mutant mitochondrial DNA: biodistribution and toxicology profile.Invest Ophthalmol Vis Sci. 2014 Oct 23;55(12):7739-53. doi: 10.1167/iovs.14-15388. Invest Ophthalmol Vis Sci. 2014. PMID: 25342621 Free PMC article.
-
Leber hereditary optic neuropathy gene therapy.Curr Opin Ophthalmol. 2024 May 1;35(3):244-251. doi: 10.1097/ICU.0000000000001028. Epub 2023 Dec 20. Curr Opin Ophthalmol. 2024. PMID: 38117686 Free PMC article. Review.
-
Juvenile open-angle Glaucoma associated with Leber's hereditary optic neuropathy: a case report and literature review.BMC Ophthalmol. 2018 Dec 17;18(1):323. doi: 10.1186/s12886-018-0980-2. BMC Ophthalmol. 2018. PMID: 30558558 Free PMC article. Review.
Cited by
-
Exogenous expression of ATP8, a mitochondrial encoded protein, from the nucleus in vivo.Mol Ther Methods Clin Dev. 2024 Nov 6;32(4):101372. doi: 10.1016/j.omtm.2024.101372. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39659757 Free PMC article.
-
Mitochondrial Transfer of the Mutant Human ND6T14484C Gene Causes Visual Loss and Optic Neuropathy.Transl Vis Sci Technol. 2020 Oct 1;9(11):1. doi: 10.1167/tvst.9.11.1. eCollection 2020 Oct. Transl Vis Sci Technol. 2020. PMID: 33101779 Free PMC article.
-
Clinical Approaches for Mitochondrial Diseases.Cells. 2023 Oct 20;12(20):2494. doi: 10.3390/cells12202494. Cells. 2023. PMID: 37887337 Free PMC article. Review.
-
Consequences of zygote injection and germline transfer of mutant human mitochondrial DNA in mice.Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):E5689-98. doi: 10.1073/pnas.1506129112. Epub 2015 Oct 5. Proc Natl Acad Sci U S A. 2015. PMID: 26438859 Free PMC article.
-
Pathway analysis identifies altered mitochondrial metabolism, neurotransmission, structural pathways and complement cascade in retina/RPE/ choroid in chick model of form-deprivation myopia.PeerJ. 2018 Jun 27;6:e5048. doi: 10.7717/peerj.5048. eCollection 2018. PeerJ. 2018. PMID: 29967729 Free PMC article.
References
-
- Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–30. - PubMed
-
- Chinnery PF, Johnson MA, Wardell TM, Singh-Kler R, Hayes C, Brown DT, Taylor RW, Bindoff LA, Turnbull DM. The epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol. 2000;48:188–93. - PubMed
-
- Sazanov LA, Peak-Chew SY, Fearnley IM, Walker JE. Resolution of the membrane domain of bovine complex I into subcomplexes: implications for the structural organization of the enzyme. Biochemistry. 2000;39:7229–35. - PubMed
-
- Howell N. Leber hereditary optic neuropathy: mitochondrial mutations and degeneration of the optic nerve. Vision Res. 1997;37:3495–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
