Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy
- PMID: 22774992
- PMCID: PMC3406377
- DOI: 10.1111/j.1365-2249.2012.04594.x
Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy
Abstract
The importance of serum immunoglobulin (Ig)G concentration in IgG replacement therapy for primary immunodeficiency diseases is established in certain settings. Generally, IgG is infused via the intravenous (IVIG) or subcutaneous (SCIG) route. For IVIG infusion, published data demonstrate that higher IgG doses and trough levels provide patients with improved protection from infection. The same conclusions are not yet accepted for SCIG; data from two recent Phase III studies and a recent post-hoc analysis, however, suggest the same correlation between higher SCIG dose and serum IgG concentration and decreased incidence of infection seen with IVIG. Other measures of clinical efficacy have not been considered similarly. Thus, combined analyses of these and other published SCIG studies were performed; a full comparison of the 13 studies was, however, limited by non-standardized definitions and reporting. Despite these limitations, our analyses indicate that certain clinical outcomes improve at higher SCIG doses and associated higher serum IgG concentrations, and suggest that there might be opportunity to improve patient outcomes via SCIG dose adjustment.
© 2012 The Authors. Clinical and Experimental Immunology © 2012 British Society for Immunology.
Figures
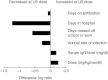
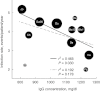
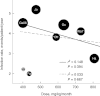
 between efficacy indicators in the US and European trials [25].
between efficacy indicators in the US and European trials [25].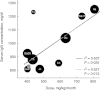
Similar articles
-
Comparative study of subcutaneous versus intravenous IgG replacement therapy in pediatric patients with primary immunodeficiency diseases: a multicenter study in Argentina.J Clin Immunol. 2013 Oct;33(7):1216-22. doi: 10.1007/s10875-013-9916-z. Epub 2013 Jul 12. J Clin Immunol. 2013. PMID: 23846854
-
Safety and efficacy of home-based subcutaneous immunoglobulin G in elderly patients with primary immunodeficiency diseases.Postgrad Med. 2011 Sep;123(5):186-93. doi: 10.3810/pgm.2011.09.2474. Postgrad Med. 2011. PMID: 21904101
-
Czech Hizentra Noninterventional Study With Rapid Push: Efficacy, Safety, Tolerability, and Convenience of Therapy With 20% Subcutaneous Immunoglobulin.Clin Ther. 2019 Nov;41(11):2231-2238. doi: 10.1016/j.clinthera.2019.08.013. Epub 2019 Sep 26. Clin Ther. 2019. PMID: 31564514 Clinical Trial.
-
Intravenous and subcutaneous immunoglobulin G replacement therapy.Allergy Asthma Proc. 2016 Nov;37(6):426-431. doi: 10.2500/aap.2016.37.3987. Allergy Asthma Proc. 2016. PMID: 27931296 Review.
-
Choices in IgG replacement therapy for primary immune deficiency diseases: subcutaneous IgG vs. intravenous IgG and selecting an optimal dose.Curr Opin Allergy Clin Immunol. 2011 Dec;11(6):532-8. doi: 10.1097/ACI.0b013e32834c22da. Curr Opin Allergy Clin Immunol. 2011. PMID: 21971330 Review.
Cited by
-
Use of subcutaneous immunoglobulin in primary immune deficiencies.Turk Pediatri Ars. 2016 Mar 1;51(1):8-14. doi: 10.5152/TurkPediatriArs.2016.3058. eCollection 2016 Mar. Turk Pediatri Ars. 2016. PMID: 27103859 Free PMC article.
-
Outcome Evaluation of a Subcutaneous Immunoglobulin Clinical Management Program.J Res Pharm Pract. 2019 Apr-Jun;8(2):52-63. doi: 10.4103/jrpp.JRPP_18_36. J Res Pharm Pract. 2019. PMID: 31367639 Free PMC article.
-
Broadening the translational immunology landscape.Clin Exp Immunol. 2012 Dec;170(3):249-53. doi: 10.1111/j.1365-2249.2012.04671.x. Clin Exp Immunol. 2012. PMID: 23121665 Free PMC article.
-
Subclinical infection and dosing in primary immunodeficiencies.Clin Exp Immunol. 2014 Dec;178 Suppl 1(Suppl 1):67-9. doi: 10.1111/cei.12516. Clin Exp Immunol. 2014. PMID: 25546767 Free PMC article. No abstract available.
-
Advances in neonatal screening for primary immune deficiencies.Exp Ther Med. 2016 May;11(5):1542-1544. doi: 10.3892/etm.2016.3119. Epub 2016 Mar 1. Exp Ther Med. 2016. PMID: 27168770 Free PMC article.
References
-
- Eijkhout HW, van der Meer JW, Kallenberg CG, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001;135:165–74. - PubMed
-
- Bonilla FA. Intravenous immunoglobulin: adverse reactions and management. J Allergy Clin Immunol. 2008;122:1238–9. - PubMed
-
- Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125:1354–60. - PubMed
-
- Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137:21–30. - PubMed

