Immune responses against islet allografts during tapering of immunosuppression--a pilot study in 5 subjects
- PMID: 22774994
- PMCID: PMC3406379
- DOI: 10.1111/j.1365-2249.2012.04605.x
Immune responses against islet allografts during tapering of immunosuppression--a pilot study in 5 subjects
Abstract
Transplantation of isolated islet of Langerhans cells has great potential as a cure for type 1 diabetes but continuous immune suppressive therapy often causes considerable side effects. Tapering of immunosuppression in successfully transplanted patients would lower patients' health risk. To identify immune biomarkers that may prove informative in monitoring tapering, we studied the effect of tapering on islet auto- and alloimmune reactivity in a pilot study in five transplant recipients in vitro. Cytokine responses to the graft were measured using Luminex technology. Avidity of alloreactive cytotoxic T Lymphocytes (CTL) was determined by CD8 blockade. The influence of immunosuppression was mimicked by in vitro replenishment of tacrolimus and MPA, the active metabolite of mycophenolate mofetil. Tapering of tacrolimus was generally followed by decreased C-peptide production. T-cell autoreactivity increased in four out of five patients during tapering. Overall alloreactive CTL precursor frequencies did not change, but their avidity to donor mismatches increased significantly after tapering (P = 0·035). In vitro addition of tacrolimus but not MPA strongly inhibited CTL alloreactivity during tapering and led to a significant shift to anti-inflammatory graft-specific cytokine production. Tapering of immunosuppression is characterized by diverse immune profiles that appear to relate inversely to plasma C-peptide levels. Highly avid allospecific CTLs that are known to associate with rejection increased during tapering, but could be countered by restoring immune suppression in vitro. Immune monitoring studies may help guiding tapering of immunosuppression after islet cell transplantation, even though we do not have formal prove yet that the observed changes reflect direct effects of immune suppression on immunity.
© 2012 The Authors. Clinical and Experimental Immunology © 2012 British Society for Immunology.
Figures

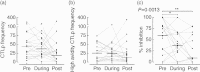

 ), with MPA (▴) or tacrolimus and MPA (▾). *P < 0·05, **P < 0·01, ***P < 0·001 by Friedman test. In all significant tests with P < 0·01, the difference between No IS and TAC/MPA remains significant after Dunn's correction for multiple comparison.
), with MPA (▴) or tacrolimus and MPA (▾). *P < 0·05, **P < 0·01, ***P < 0·001 by Friedman test. In all significant tests with P < 0·01, the difference between No IS and TAC/MPA remains significant after Dunn's correction for multiple comparison.
 ), with MPA (▴) or tacrolimus and MPA (▾). *P < 0·05, **P < 0·01, ***P < 0·001 by Friedman test. For all comparisons significant differences remain after Dunn's correction for multiple comparison: for IL10, pre and post tapering No IS – tacrolimus and MPA – tacrolimus and during tapering MPA – tacrolimus/MPA; for IL2, pre tapering No IS – tacrolimus, during tapering No IS – tacrolimus and MPA – tacrolimus and post tapering MPA – tacrolimus/MPA remain significant. For TNFα during tapering No IS – tacrolimus.
), with MPA (▴) or tacrolimus and MPA (▾). *P < 0·05, **P < 0·01, ***P < 0·001 by Friedman test. For all comparisons significant differences remain after Dunn's correction for multiple comparison: for IL10, pre and post tapering No IS – tacrolimus and MPA – tacrolimus and during tapering MPA – tacrolimus/MPA; for IL2, pre tapering No IS – tacrolimus, during tapering No IS – tacrolimus and MPA – tacrolimus and post tapering MPA – tacrolimus/MPA remain significant. For TNFα during tapering No IS – tacrolimus.References
-
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. - PubMed
-
- Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–68. - PubMed
-
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–30. - PubMed
-
- Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

