Structural mechanism for alteration of collagen gel mechanics by glutaraldehyde crosslinking
- PMID: 22775003
- PMCID: PMC3825191
- DOI: 10.3109/03008207.2011.640760
Structural mechanism for alteration of collagen gel mechanics by glutaraldehyde crosslinking
Abstract
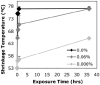
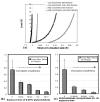
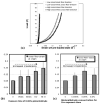
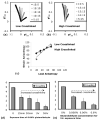
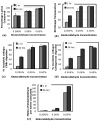
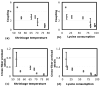
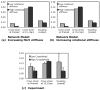
Soft collagenous tissues that are loaded in vivo undergo crosslinking during aging and wound healing. Bioprosthetic tissues implanted in vivo are also commonly crosslinked with glutaraldehyde (GA). While crosslinking changes the mechanical properties of the tissue, the nature of the mechanical changes and the underlying microstructural mechanism are poorly understood. In this study, a combined mechanical, biochemical and simulation approach was employed to identify the microstructural mechanism by which crosslinking alters mechanical properties. The model collagenous tissue used was an anisotropic cell-compacted collagen gel, and the model crosslinking agent was monomeric GA. The collagen gels were incrementally crosslinked by either increasing the GA concentration or increasing the crosslinking time. In biaxial loading experiments, increased crosslinking produced (1) decreased strain response to a small equibiaxial preload, with little change in response to subsequent loading and (2) decreased coupling between the fiber and cross-fiber direction. The mechanical trend was found to be better described by the lysine consumption data than by the shrinkage temperature. The biaxial loading of incrementally crosslinked collagen gels was simulated computationally with a previously published network model. Crosslinking was represented by increased fibril stiffness or by increased resistance to fibril rotation. Only the latter produced mechanical trends similar to that observed experimentally. Representing crosslinking as increased fibril stiffness did not reproduce the decreased coupling between the fiber and cross-fiber directions. The study concludes that the mechanical changes in crosslinked collagen gels are caused by the microstructural mechanism of increased resistance to fibril rotation.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures

References
-
- Brinckmann J, Notbohm H, Müller PK, editors. Collagen - primer in structure, processing and assembly. Springer; 2005.
-
- Stenzel KH, Miyata T, Rubin AL. Collagen as a biomaterial. Annual Review of Biophysics and Bioengineering. 1974;3(1):231–53. - PubMed
-
- Eyre DR, Paz MA, Gallop PM. Cross-Linking in Collagen and Elastin. Annual Review of Biochemistry. 1984;53(1):717–48. - PubMed
-
- McCormick RJ, Thomas DP. Collagen crosslinking in the heart: relationship to development and function. Basic Appl Myol. 1998;8(2):143–50.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
