GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence
- PMID: 22776039
- PMCID: PMC3556522
- DOI: 10.1111/j.1463-1326.2012.01663.x
GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence
Abstract
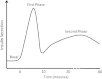
The major goal in the treatment of type 2 diabetes mellitus is to control the hyperglycaemia characteristic of the disease. However, treatment with common therapies such as insulin or insulinotrophic sulphonylureas (SU), while effective in reducing hyperglycaemia, may impose a greater risk of hypoglycaemia, as neither therapy is self-regulated by ambient blood glucose concentrations. Hypoglycaemia has been associated with adverse physical and psychological outcomes and may contribute to negative cardiovascular events; hence minimization of hypoglycaemia risk is clinically advantageous. Stimulation of insulin secretion from pancreatic β-cells by glucagon-like peptide 1 receptor (GLP-1R) agonists is known to be glucose-dependent. GLP-1R agonists potentiate glucose-stimulated insulin secretion and have little or no activity on insulin secretion in the absence of elevated blood glucose concentrations. This 'glucose-regulated' activity of GLP-1R agonists makes them useful and potentially safer therapeutics for overall glucose control compared to non-regulated therapies; hyperglycaemia can be reduced with minimal hypoglycaemia. While the inherent mechanism of action of GLP-1R agonists mediates their glucose dependence, studies in rats suggest that SUs may uncouple this dependence. This hypothesis is supported by clinical studies showing that the majority of events of hypoglycaemia in patients treated with GLP-1R agonists occur in patients treated with a concomitant SU. This review aims to discuss the current understanding of the mechanisms by which GLP-1R signalling promotes insulin secretion from pancreatic β-cells via a glucose-dependent process.
© 2012 Blackwell Publishing Ltd.
Figures
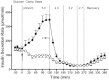
 , exenatide treatment arm. Data are means ± s.e.; n = 11 per treatment arm. *p < 0.05, exenatide vs. placebo during steady state of a glycaemic interval. Reproduced with permission from Degn et al. [31].
, exenatide treatment arm. Data are means ± s.e.; n = 11 per treatment arm. *p < 0.05, exenatide vs. placebo during steady state of a glycaemic interval. Reproduced with permission from Degn et al. [31].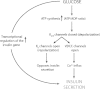
Similar articles
-
Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors.Diabetes Obes Metab. 2016 Mar;18(3):203-16. doi: 10.1111/dom.12591. Epub 2016 Jan 5. Diabetes Obes Metab. 2016. PMID: 26489970 Free PMC article. Review.
-
The pharmacologic basis for clinical differences among GLP-1 receptor agonists and DPP-4 inhibitors.Postgrad Med. 2011 Nov;123(6):189-201. doi: 10.3810/pgm.2011.11.2508. Postgrad Med. 2011. PMID: 22104467
-
An overview of once-weekly glucagon-like peptide-1 receptor agonists--available efficacy and safety data and perspectives for the future.Diabetes Obes Metab. 2011 May;13(5):394-407. doi: 10.1111/j.1463-1326.2011.01357.x. Epub 2011 Jan 5. Diabetes Obes Metab. 2011. PMID: 21208359 Review.
-
Incretins: their physiology and application in the treatment of diabetes mellitus.Diabetes Metab Res Rev. 2014 Jul;30(5):354-71. doi: 10.1002/dmrr.2501. Diabetes Metab Res Rev. 2014. PMID: 24989141 Review.
-
Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonists and DPP-4 inhibitors.J Am Pharm Assoc (2003). 2009 Sep-Oct;49 Suppl 1:S16-29. doi: 10.1331/JAPhA.2009.09078. J Am Pharm Assoc (2003). 2009. PMID: 19801361 Review.
Cited by
-
Evaluation of Characteristics of Gastrointestinal Adverse Events with Once-Weekly Dulaglutide Treatment in Chinese Patients with Type 2 Diabetes: A Post Hoc Pooled Analysis of Two Randomized Trials.Diabetes Ther. 2020 Aug;11(8):1821-1833. doi: 10.1007/s13300-020-00869-z. Epub 2020 Jul 4. Diabetes Ther. 2020. PMID: 32621083 Free PMC article.
-
Brexpiprazole caused glycolipid metabolic disorder by inhibiting GLP1/GLP1R signaling in rats.Acta Pharmacol Sin. 2021 Aug;42(8):1267-1279. doi: 10.1038/s41401-021-00680-x. Epub 2021 May 11. Acta Pharmacol Sin. 2021. PMID: 33976388 Free PMC article.
-
Mechanism of cardiovascular disease benefit of glucagon-like peptide 1 agonists.Cardiovasc Endocrinol Metab. 2018 Feb 14;7(1):18-23. doi: 10.1097/XCE.0000000000000147. eCollection 2018 Mar. Cardiovasc Endocrinol Metab. 2018. PMID: 31646274 Free PMC article. Review.
-
Butyrate to combat obesity and obesity-associated metabolic disorders: Current status and future implications for therapeutic use.Obes Rev. 2022 Oct;23(10):e13498. doi: 10.1111/obr.13498. Epub 2022 Jul 20. Obes Rev. 2022. PMID: 35856338 Free PMC article. Review.
-
Enteroendocrine cells: a review of their role in brain-gut communication.Neurogastroenterol Motil. 2016 May;28(5):620-30. doi: 10.1111/nmo.12754. Epub 2015 Dec 21. Neurogastroenterol Motil. 2016. PMID: 26691223 Free PMC article. Review.
References
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1577–1596. - PubMed
-
- Pettersson B, Rosenqvist U, Deleskog A, Journath G, Wändell P. Self-reported experience of hypoglycemia among adults with type 2 diabetes mellitus (Exhype) Diabetes Res Clin Pract. 2011;92:19–25. - PubMed
-
- Eckert B, Agardh CD. Hypoglycaemia leads to an increased QT interval in normal men. Clin Physiol. 1998;18:570–575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

