Time-dependent effects of hypothermia on microglial activation and migration
- PMID: 22776061
- PMCID: PMC3470995
- DOI: 10.1186/1742-2094-9-164
Time-dependent effects of hypothermia on microglial activation and migration
Abstract
Background: Therapeutic hypothermia is one of the neuroprotective strategies that improve neurological outcomes after brain damage in ischemic stroke and traumatic brain injury. Microglial cells become activated following brain injury and play an important role in neuroinflammation and subsequent brain damage. The aim of this study was to determine the time-dependent effects of hypothermia on microglial cell activation and migration, which are accompanied by neuroinflammation.
Methods: Microglial cells in culture were subjected to mild (33 °C) or moderate (29 °C) hypothermic conditions before, during, or after lipopolysaccharide (LPS) or hypoxic stimulation, and the production of nitric oxide (NO), proinflammatory cytokines, reactive oxygen species, and neurotoxicity was evaluated. Effects of hypothermia on microglial migration were also determined in in vitro as well as in vivo settings.
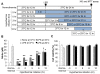
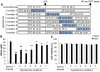
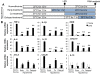
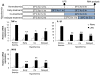
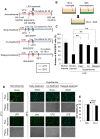
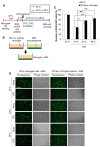
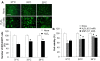
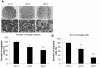
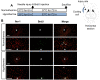
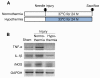
Results: Early-, co-, and delayed-hypothermic treatments inhibited microglial production of inflammatory mediators to varying degrees: early treatment was the most efficient, and delayed treatment showed time-dependent effects. Delayed hypothermia also suppressed the mRNA levels of proinflammatory cytokines and iNOS, and attenuated microglial neurotoxicity in microglia-neuron co-cultures. Furthermore, delayed hypothermia reduced microglial migration in the Boyden chamber assay and wound healing assay. In a stab injury model, delayed local hypothermia reduced migration of microglia toward the injury site in the rat brain.
Conclusion: Taken together, our results indicate that delayed hypothermia is sufficient to attenuate microglial activation and migration, and provide the basis of determining the optimal time window for therapeutic hypothermia. Delayed hypothermia may be neuroprotective by inhibiting microglia-mediated neuroinflammation, indicating the therapeutic potential of post-injury hypothermia for patients with brain damages exhibiting some of the inflammatory components.
Figures









References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

