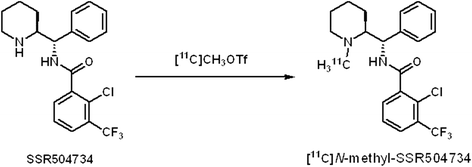
Synthesis and evaluation of 2-chloro N-[(S)-{(S)-1-[11 C]methylpiperidin-2-yl} (phenyl)methyl]3-trifluoromethyl-benzamide ([11 C]N-methyl-SSR504734) as a PET radioligand for glycine transporter 1
- PMID: 22776065
- PMCID: PMC3531252
- DOI: 10.1186/2191-219X-2-37
Synthesis and evaluation of 2-chloro N-[(S)-{(S)-1-[11 C]methylpiperidin-2-yl} (phenyl)methyl]3-trifluoromethyl-benzamide ([11 C]N-methyl-SSR504734) as a PET radioligand for glycine transporter 1
Abstract
Background: Dysfunction of the glycine transporter 1 (GlyT1) has been suggested to be involved in psychiatric disorders such as schizophrenia. GlyT1 inhibitors have therefore been considered to have antipsychotic therapeutic potential. Positron emission tomography (PET) imaging probes for GlyT1 are, consequently, expected to be useful for investigating the mechanism of such disease conditions and for measuring occupancy of GlyT1 inhibitors in vivo. The aim of this study was to assess the potential of 2-chloro N-[(S)-{(S)-1-[11 C]methylpiperidin-2-yl} (phenyl)methyl] 3-trifluoromethyl-benzamide ([11 C]N-methyl-SSR504734) as a PET imaging agent for GlyT1.
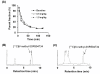
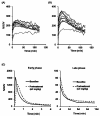
Methods: [11 C]N-methyl-SSR504734 was synthesized by N-[11 C]methylation of SSR504734 via [11 C]CH3OTf. In vitro brain distribution of [11 C]N-methyl-SSR504734 was tested in whole-hemisphere autoradiography (ARG) on human brain slices. Initial PET studies were performed using a cynomolgus monkey at baseline and after pretreatment with 0.1 to 1.5 mg/kg of SSR504734. Then, PET studies using rhesus monkeys were performed with arterial blood sampling at baseline and after pretreatment with 1.5 to 4.5 mg/kg SSR504734. Distribution volumes (VT) were calculated with a two-tissue compartment model, and GlyT1 occupancy by SSR504734 was estimated using a Lassen plot approach.
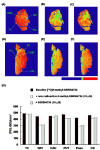
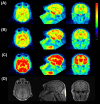
Results: [11 C]N-methyl-SSR504734 was successfully synthesized in moderate radiochemical yield and high specific radioactivity. In the ARG experiments, [11 C]N-methyl-SSR504734 showed specific binding in the white matter and pons. In the initial PET experiments in a cynomolgus monkey, [11 C]N-methyl-SSR504734 showed high brain uptake and consistent distribution with previously reported GlyT1 expression in vivo (thalamus, brainstem > cerebellum > cortical regions). However, the brain uptake increased after pretreatment with SSR504734. Further PET studies in rhesus monkeys showed a similar increase of brain uptake after pretreatment with SSR504734. However, the VT of [11 C]N-methyl-SSR504734 was found to decrease after pretreatment of SSR504734 in a dose-dependent manner. GlyT1 occupancy was calculated to be 45% and 73% at 1.5 and 4.5 mg/kg of SSR504734, respectively.
Conclusions: [11 C]N-methyl-SSR504734 is demonstrated to be a promising PET radioligand for GlyT1 in nonhuman primates. The present results warrant further PET studies in human subjects.
Figures





References
-
- Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–564. - PubMed
-
- Jursky F, Nelson N. Localization of glycine neurotransmitter transporter (GLYT2) reveals correlation with the distribution of glycine receptor. J Neurochem. 1995;64:1026–1033. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

