Whole genome expression profile in neuroblastoma cells exposed to 1-methyl-4-phenylpyridine
- PMID: 22776087
- PMCID: PMC3470775
- DOI: 10.1016/j.neuro.2012.06.009
Whole genome expression profile in neuroblastoma cells exposed to 1-methyl-4-phenylpyridine
Abstract
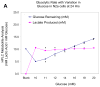
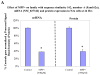
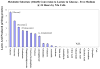
Mitochondrial dysfunction and subsequent energy failure is a contributing factor to degeneration of the substantia nigra pars compacta associated with Parkinson's disease (PD). In this study, we investigate molecular events triggered by cell exposure to the mitochondrial toxin 1-methyl-4-phenylpyridine (MPP+) using whole genome-expression microarray, Western Blot and metabolic studies. The data show that MPP+ (500 μM) obstructs mitochondrial respiration/oxidative phosphorylation (OXPHOS) in mouse neuroblastoma Neuro-2a cells, juxtaposing accelerated glucose consumption and production of lactic acid. While additional glucose concentrations restored viability in the presence of MPP+ (500 μM), the loss of OXPHOS was sustained, suggesting that compensatory anaerobic metabolic systems were fulfilling required energy needs. Under these conditions, MPP+ initiated significant changes to the transcription of 439 genes of which 287 DAVID IDs were identified and subsequent functional annotation clusters identified. Prominent changes were as follows; MPP+ initiated loss of mRNA for mitochondrial encoded 3-hydroxybutyratedehydrogenase, type 2(Bdh2), tv1, NADH dehydrogenase 4,5 genes, cytochrome b and NADH dehydrogenase (ubiquinone) flavoprotein 3, concomitant to rise in a mitochondrial fission gene; ganglioside-induced differentiation-associated-protein 1 (GDAP1). The negative changes to OXPHOS components were accompanied by protective forces within the mitochondria espousing elevated ratio of anti/pro-apoptotic processes. These included a loss of apoptotic Bcl-2/adenovirus E1B 19-kDa-interacting protein (BNIP3) and family with sequence similarity 162, member A (FAM162a) and rise of heat shock protein 1 and Lon peptidase 1. There were no changes indicative of free radical damage (e.g. SOD, GSH-Px), rather MPP+ initiated significant elevation in G protein signaling components (which trigger catabolic processes) and anaerobic metabolic systems involving carboxylic acid/transamination reactions (e.g. glutamate oxaloacetate transaminase 1 (GOT1), glutamic pyruvate-alanine transaminase 2 (GPT2), cystathionase and redox proteins such as cytochrome b5 reductase 1 and ferredoxin reductase. Counter-intuitively, the data show reduction of mRNA in glycolytic processes [DAVID enrichment score 9.96 p value 1.90E-19], some corroborated by Western Blot, bringing in to question the sources of lactate observed in the presence of MPP+. Examining this aspect, the data show that diverse carboxylic acids (succinate, oxaloacetate and a-ketoglutarate) are capable of contributing to the lactate pool in addition to phosph(enolpyruvate) or pyruvate in the absence of glucose by this cell line. In conclusion, these findings show that MPP+ negatively affects the transcriptome involved with complex I, but initiated an elevation of G protein signaling and anaerobic metabolic systems involved with nitrogen/carboxylic acid metabolism. Future research will be required to elucidate the survival pathways that drive anaerobic substrate level phosphorylation, and define functional ramification to the loss of mitochondrial FAM162a and BNIP3 proteins.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures








References
-
- Absi E, Ayala A, Machado A, Parrado J. Protective effect of melatonin against the 1-methyl-4-phenylpyridinium-induced inhibition of complex I of the mitochondrial respiratory chain. J Pineal Res. 2000;29:40–7. - PubMed
-
- Akashi S, Kimura T, Takeuchi T, Kuramochi K, Kobayashi S, Sugawara F, et al. Neoechinulin a impedes the progression of rotenone-induced cytotoxicity in PC12 cells. Biol Pharm Bull. 2011;34:243–8. - PubMed
-
- Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17:353–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

