Mesenchymal stem cells for cartilage repair in osteoarthritis
- PMID: 22776206
- PMCID: PMC3580463
- DOI: 10.1186/scrt116
Mesenchymal stem cells for cartilage repair in osteoarthritis
Abstract
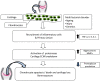
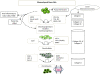
Osteoarthritis (OA) is a degenerative disease of the connective tissue and progresses with age in the older population or develops in young athletes following sports-related injury. The articular cartilage is especially vulnerable to damage and has poor potential for regeneration because of the absence of vasculature within the tissue. Normal load-bearing capacity and biomechanical properties of thinning cartilage are severely compromised during the course of disease progression. Although surgical and pharmaceutical interventions are currently available for treating OA, restoration of normal cartilage function has been difficult to achieve. Since the tissue is composed primarily of chondrocytes distributed in a specialized extracellular matrix bed, bone marrow stromal cells (BMSCs), also known as bone marrow-derived 'mesenchymal stem cells' or 'mesenchymal stromal cells', with inherent chondrogenic differentiation potential appear to be ideally suited for therapeutic use in cartilage regeneration. BMSCs can be easily isolated and massively expanded in culture in an undifferentiated state for therapeutic use. Owing to their potential to modulate local microenvironment via anti-inflammatory and immunosuppressive functions, BMSCs have an additional advantage for allogeneic application. Moreover, by secreting various bioactive soluble factors, BMSCs can protect the cartilage from further tissue destruction and facilitate regeneration of the remaining progenitor cells in situ. This review broadly describes the advances made during the last several years in BMSCs and their therapeutic potential for repairing cartilage damage in OA.
Figures
References
-
- Poole AR. In: Arthrities and Allied Conditions: A Textbook of Rheumatology. 13. Koopman WS, editor. Baltimore Lippincott Williams and Wilkins; 1997. Cartilage in health and disease; pp. 255–308.
-
- Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004. pp. S6–S15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

