Necdin, a p53 target gene, regulates the quiescence and response to genotoxic stress of hematopoietic stem/progenitor cells
- PMID: 22776820
- PMCID: PMC3429304
- DOI: 10.1182/blood-2011-11-393983
Necdin, a p53 target gene, regulates the quiescence and response to genotoxic stress of hematopoietic stem/progenitor cells
Abstract
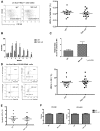
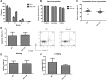
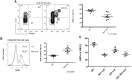
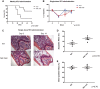
We recently defined a critical role for p53 in regulating the quiescence of adult hematopoietic stem cells (HSCs) and identified necdin as a candidate p53 target gene. Necdin is a growth-suppressing protein and the gene encoding it is one of several that are deleted in patients with Prader-Willi syndrome. To define the intrinsic role of necdin in adult hematopoiesis, in the present study, we transplanted necdin-null fetal liver cells into lethally irradiated recipients. We show that necdin-null adult HSCs are less quiescent and more proliferative than normal HSCs, demonstrating the similar role of necdin and p53 in promoting HSC quiescence during steady-state conditions. However, wild-type recipients repopulated with necdin-null hematopoietic stem/progenitor cells show enhanced sensitivity to irradiation and chemotherapy, with increased p53-dependent apoptosis, myelosuppression, and mortality. Necdin controls the HSC response to genotoxic stress via both cell-cycle-dependent and cell-cycle-independent mechanisms, with the latter occurring in a Gas2L3-dependent manner. We conclude that necdin functions as a molecular switch in adult hematopoiesis, acting in a p53-like manner to promote HSC quiescence in the steady state, but suppressing p53-dependent apoptosis in response to genotoxic stress.
Figures


References
-
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2):115–128. - PubMed
-
- Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. - PubMed
-
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

