S-Nitrosation of β-catenin and p120 catenin: a novel regulatory mechanism in endothelial hyperpermeability
- PMID: 22777005
- PMCID: PMC3966064
- DOI: 10.1161/CIRCRESAHA.112.274548
S-Nitrosation of β-catenin and p120 catenin: a novel regulatory mechanism in endothelial hyperpermeability
Abstract
Rationale: Endothelial adherens junction proteins constitute an important element in the control of microvascular permeability. Platelet-activating factor (PAF) increases permeability to macromolecules via translocation of endothelial nitric oxide synthase (eNOS) to cytosol and stimulation of eNOS-derived nitric oxide signaling cascade. The mechanisms by which nitric oxide signaling regulates permeability at adherens junctions are still incompletely understood.
Objective: We explored the hypothesis that PAF stimulates hyperpermeability via S-nitrosation (SNO) of adherens junction proteins.
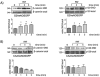
Methods and results: We measured PAF-stimulated SNO of β-catenin and p120-catenin (p120) in 3 cell lines: ECV-eNOSGFP, EAhy926 (derived from human umbilical vein), and postcapillary venular endothelial cells (derived from bovine heart endothelium) and in the mouse cremaster muscle in vivo. SNO correlated with diminished abundance of β-catenin and p120 at the adherens junction and with hyperpermeability. Tumor necrosis factor-α increased nitric oxide production and caused similar increase in SNO as PAF. To ascertain the importance of eNOS subcellular location in this process, we used ECV-304 cells transfected with cytosolic eNOS (GFPeNOSG2A) and plasma membrane eNOS (GFPeNOSCAAX). PAF induced SNO of β-catenin and p120 and significantly diminished association between these proteins in cells with cytosolic eNOS but not in cells wherein eNOS is anchored to the cell membrane. Inhibitors of nitric oxide production and of SNO blocked PAF-induced SNO and hyperpermeability, whereas inhibition of the cGMP pathway had no effect. Mass spectrometry analysis of purified p120 identified cysteine 579 as the main S-nitrosated residue in the region that putatively interacts with vascular endothelial-cadherin.
Conclusions: Our results demonstrate that agonist-induced SNO contributes to junctional membrane protein changes that enhance endothelial permeability.
Figures



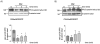



References
-
- Ramirez MM, Quardt SM, Kim D, Oshiro H, Minnicozzi M, Duran WN. Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvascular research. 1995;50:223–234. - PubMed
-
- Yuan SY. New insights into eNOS signaling in microvascular permeability. American journal of physiology. Heart and circulatory physiology. 2006;291:H1029–1031. - PubMed
-
- Mayhan WG. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5’-diphosphate and bradykinin. Inflammation. 1992;16:295–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

