LRP1-dependent endocytic mechanism governs the signaling output of the bmp system in endothelial cells and in angiogenesis
- PMID: 22777006
- PMCID: PMC3495066
- DOI: 10.1161/CIRCRESAHA.112.274597
LRP1-dependent endocytic mechanism governs the signaling output of the bmp system in endothelial cells and in angiogenesis
Abstract
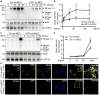
Rationale: Among the extracellular modulators of Bmp (bone morphogenetic protein) signaling, Bmper (Bmp endothelial cell precursor-derived regulator) both enhances and inhibits Bmp signaling. Recently we found that Bmper modulates Bmp4 activity via a concentration-dependent, endocytic trap-and-sink mechanism.
Objective: To investigate the molecular mechanisms required for endocytosis of the Bmper/Bmp4 and signaling complex and determine the mechanism of Bmper's differential effects on Bmp4 signaling.
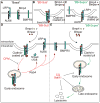
Methods and results: Using an array of biochemical and cell biology techniques, we report that LRP1 (LDL receptor-related protein 1), a member of the LDL receptor family, acts as an endocytic receptor for Bmper and a coreceptor of Bmp4 to mediate the endocytosis of the Bmper/Bmp4 signaling complex. Furthermore, we demonstrate that LRP1-dependent Bmper/Bmp4 endocytosis is essential for Bmp4 signaling, as evidenced by the phenotype of lrp1-deficient zebrafish, which have abnormal cardiovascular development and decreased Smad1/5/8 activity in key vasculogenic structures.
Conclusions: Together, these data reveal a novel role for LRP1 in the regulation of Bmp4 signaling by regulating receptor complex endocytosis. In addition, these data introduce LRP1 as a critical regulator of vascular development. These observations demonstrate Bmper's ability to fine-tune Bmp4 signaling at the single-cell level, unlike the spatial regulatory mechanisms applied by other Bmp modulators.
Figures




References
-
- Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103:804–812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

