Fluoro-Sorafenib (Regorafenib) effects on hepatoma cells: growth inhibition, quiescence, and recovery
- PMID: 22777740
- PMCID: PMC4509637
- DOI: 10.1002/jcp.24148
Fluoro-Sorafenib (Regorafenib) effects on hepatoma cells: growth inhibition, quiescence, and recovery
Abstract
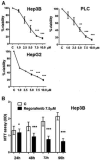
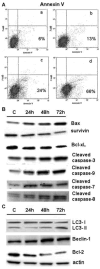
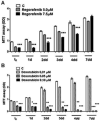
To evaluate the growth-inhibitory properties of the potent multi-kinase antagonist Regorafenib (Fluoro-Sorafenib), which was synthesized as a more potent Sorafenib, a Raf inhibitor and to determine whether similar mechanisms were involved, human hepatoma cell lines were grown in the presence or absence of Regorafanib and examined for growth inhibition. Western blots were performed for Raf targets, apoptosis, and autophagy. Regorafenib inhibited growth of human Hep3B, PLC/PRF/5, and HepG2 cells in a concentration- and time-dependent manner. Multiple signaling pathways were altered, including MAP kinases phospho-ERK and phospho-JNK and its target phospho-c-Jun. There was evidence for apoptosis by FACS, cleavage of caspases and increased Bax levels; as well as induction of autophagy, as judged by increased Beclin-1 and LC3 (II) levels. Prolonged drug exposure resulted in cell quiescence. Full growth recovery occurred after drug removal, unlike with doxorubicin chemotherapy. Regorafenib is a potent inhibitor of cell growth. Cells surviving Regorafenib treatment remain viable, but quiescent and capable of regrowth following drug removal. The reversibility of tumor cell growth suppression after drug removal may have clinical implications.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest Statement: None declared.
Figures

References
-
- Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed
-
- Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2011;13:7–12. - PubMed
-
- Dumas J, Sibley R, Riedl B, Monahan MK, Lee W, Lowinger TB, Redman AM, Johnson JS, Kingery-Wood J, Scott WJ, Smith RA, Bobko M, Schoenleber R, Ranges GE, Housley TJ, Bhargava A, Wilhelm SM, Shrikhande A. Discovery of a new class of p38 kinase inhibitors. Bioorg Med Chem Lett. 2000;10:2047–2050. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

