VEGF/neuropilin-2 regulation of Bmi-1 and consequent repression of IGF-IR define a novel mechanism of aggressive prostate cancer
- PMID: 22777769
- PMCID: PMC4205964
- DOI: 10.1158/2159-8290.CD-12-0085
VEGF/neuropilin-2 regulation of Bmi-1 and consequent repression of IGF-IR define a novel mechanism of aggressive prostate cancer
Abstract
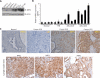
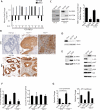
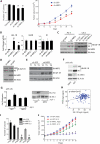
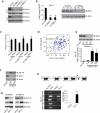
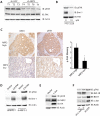
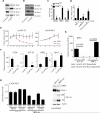
We show that the VEGF receptor neuropilin-2 (NRP2) is associated with high-grade, PTEN-null prostate cancer and that its expression in tumor cells is induced by PTEN loss as a consequence of c-Jun activation. VEGF/NRP2 signaling represses insulin-like growth factor-1 receptor (IGF-IR) expression and signaling, and the mechanism involves Bmi-1-mediated transcriptional repression of the IGF-IR. This mechanism has significant functional and therapeutic implications that were evaluated. IGF-IR expression positively correlates with PTEN and inversely correlates with NRP2 in prostate tumors. NRP2 is a robust biomarker for predicting response to IGF-IR therapy because prostate carcinomas that express NRP2 exhibit low levels of IGF-IR. Conversely, targeting NRP2 is only modestly effective because NRP2 inhibition induces compensatory IGF-IR signaling. Inhibition of both NRP2 and IGF-IR, however, completely blocks tumor growth in vivo.
Figures

References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. - PubMed
-
- Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–40. - PubMed
-
- Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–79. - PubMed
-
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

