The role of conformational flexibility in β2-microglobulin amyloid fibril formation at neutral pH
- PMID: 22777780
- PMCID: PMC3568905
- DOI: 10.1002/rcm.6282
The role of conformational flexibility in β2-microglobulin amyloid fibril formation at neutral pH
Abstract
Rationale: Amyloid formation is implicated in a number of human diseases. β(2)-Microglobulin (β(2)m) is the precursor protein in dialysis-related amyloidosis and it has been shown that partial, or more complete, unfolding is key to amyloid fibril formation in this pathology. Here the relationship between conformational flexibility and β(2)m amyloid formation at physiological pH has been investigated.
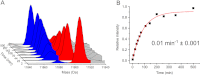
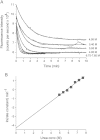
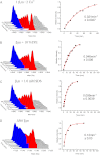
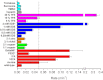
Methods: HDX-ESI-MS was used to study the conformational dynamics of β(2)m. Protein engineering, or the addition of Cu(2+) ions, sodium dodecyl sulphate, trifluoroethanol, heparin, or protein stabilisers, was employed to perturb the conformational dynamics of β(2)m. The fibril-forming propensities of the protein variants and the wild-type protein in the presence of additives, which resulted in >5-fold increase in the EX1 rate of HDX, were investigated further.
Results: ESI-MS revealed that HDX occurs via a mixed EX1/EX2 mechanism under all conditions. Urea denaturation and tryptophan fluorescence indicated that EX1 exchange occurred from a globally unfolded state in wild-type β(2)m. Although >30-fold increase in the HDX exchange rate was observed both for the protein variants and for the wild-type protein in the presence of specific additives, large increases in exchange rate did not necessarily result in extensive de novo fibril formation.
Conclusions: The conformational dynamics measured by the EX1 rate of HDX do not predict the ability of β(2)m to form amyloid fibrils de novo at neutral pH. This suggests that the formation of amyloid fibrils from β(2)m at neutral pH is dependent on the generation of one or more specific aggregation-competent species which facilitate self-assembly.
Copyright © 2012 John Wiley & Sons, Ltd.
Figures

Similar articles
-
Low concentrations of sodium dodecyl sulfate induce the extension of beta 2-microglobulin-related amyloid fibrils at a neutral pH.Biochemistry. 2004 Aug 31;43(34):11075-82. doi: 10.1021/bi049262u. Biochemistry. 2004. PMID: 15323566
-
HDX-ESI-MS reveals enhanced conformational dynamics of the amyloidogenic protein beta(2)-microglobulin upon release from the MHC-1.J Am Soc Mass Spectrom. 2009 Feb;20(2):278-86. doi: 10.1016/j.jasms.2008.10.005. Epub 2008 Oct 17. J Am Soc Mass Spectrom. 2009. PMID: 18996721 Free PMC article.
-
Ultrasonication-induced amyloid fibril formation of beta2-microglobulin.J Biol Chem. 2005 Sep 23;280(38):32843-8. doi: 10.1074/jbc.M506501200. Epub 2005 Jul 25. J Biol Chem. 2005. PMID: 16046408
-
Molecular interactions in the formation and deposition of beta2-microglobulin-related amyloid fibrils.Amyloid. 2005 Mar;12(1):15-25. doi: 10.1080/13506120500032352. Amyloid. 2005. PMID: 16076607 Review.
-
Kinetic analysis of the polymerization and depolymerization of beta(2)-microglobulin-related amyloid fibrils in vitro.Biochim Biophys Acta. 2005 Nov 10;1753(1):34-43. doi: 10.1016/j.bbapap.2005.07.007. Epub 2005 Jul 26. Biochim Biophys Acta. 2005. PMID: 16084781 Review.
Cited by
-
AGGRESCAN3D (A3D): server for prediction of aggregation properties of protein structures.Nucleic Acids Res. 2015 Jul 1;43(W1):W306-13. doi: 10.1093/nar/gkv359. Epub 2015 Apr 16. Nucleic Acids Res. 2015. PMID: 25883144 Free PMC article.
-
Nucleobindin-2 consists of two structural components: The Zn2+-sensitive N-terminal half, consisting of nesfatin-1 and -2, and the Ca2+-sensitive C-terminal half, consisting of nesfatin-3.Comput Struct Biotechnol J. 2021 Jul 30;19:4300-4318. doi: 10.1016/j.csbj.2021.07.036. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34429849 Free PMC article.
-
Distinguishing closely related amyloid precursors using an RNA aptamer.J Biol Chem. 2014 Sep 26;289(39):26859-26871. doi: 10.1074/jbc.M114.595066. Epub 2014 Aug 6. J Biol Chem. 2014. PMID: 25100729 Free PMC article.
-
Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update.Toxins (Basel). 2018 Jan 8;10(1):33. doi: 10.3390/toxins10010033. Toxins (Basel). 2018. PMID: 29316724 Free PMC article. Review.
-
Expanding the repertoire of amyloid polymorphs by co-polymerization of related protein precursors.J Biol Chem. 2013 Mar 8;288(10):7327-37. doi: 10.1074/jbc.M112.447524. Epub 2013 Jan 17. J Biol Chem. 2013. PMID: 23329840 Free PMC article.
References
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333. - PubMed
-
- Eichner T, Radford SE. A diversity of assembly mechanisms of a generic amyloid fold. Mol. Cell. 2011;43:8. - PubMed
-
- McCutchen SL, Lai Z, Miroy GJ, Kelly JW, Colon W. Comparison of lethal and nonlethal transthyretin variants and their relationship to amyloid disease. Biochemistry. 1995;34:13527. - PubMed
-
- Merlini G, Bellotti V. Lysozyme: A paradigmatic molecule for the investigation of protein structure, function and misfolding. Clin. Chim. Acta. 2005;357:168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

