Infection with Mycobacterium avium subsp. paratuberculosis results in rapid interleukin-1β release and macrophage transepithelial migration
- PMID: 22778093
- PMCID: PMC3418758
- DOI: 10.1128/IAI.06322-11
Infection with Mycobacterium avium subsp. paratuberculosis results in rapid interleukin-1β release and macrophage transepithelial migration
Abstract
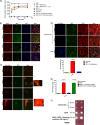
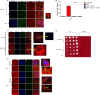
Pathogen processing by the intestinal epithelium involves a dynamic innate immune response initiated by pathogen-epithelial cell cross talk. Interactions between epithelium and Mycobacterium avium subsp. paratuberculosis have not been intensively studied, and it is currently unknown how the bacterium-epithelial cell cross talk contributes to the course of infection. We hypothesized that M. avium subsp. paratuberculosis harnesses host responses to recruit macrophages to the site of infection to ensure its survival and dissemination. We investigated macrophage recruitment in response to M. avium subsp. paratuberculosis using a MAC-T bovine macrophage coculture system. We show that M. avium subsp. paratuberculosis infection led to phagosome acidification within bovine epithelial (MAC-T) cells as early as 10 min, which resulted in upregulation of interleukin-1β (IL-1β) at transcript and protein levels. Within 10 min of infection, macrophages were recruited to the apical side of MAC-T cells. Inhibition of phagosome acidification or IL-1β abrogated this response, while MCP-1/CCL-2 blocking had no effect. IL-1β processing was dependent upon Ca(2+) uptake from the extracellular medium and intracellular Ca(2+) oscillations, as determined by EGTA and BAPTA-AM [1,2-bis(2-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid tetrakis (acetoxymethyl ester)] treatments. Thus, M. avium subsp. paratuberculosis is an opportunist that takes advantage of extracellular Ca(2+)-dependent phagosome acidification and IL-1β processing in order to efficiently transverse the epithelium and enter its niche--the macrophage.
Figures

Similar articles
-
Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium.Infect Immun. 2002 Oct;70(10):5556-61. doi: 10.1128/IAI.70.10.5556-5561.2002. Infect Immun. 2002. PMID: 12228282 Free PMC article.
-
A critical role of interleukin-10 in the response of bovine macrophages to infection by Mycobacterium avium subsp paratuberculosis.Am J Vet Res. 2005 Apr;66(4):721-6. doi: 10.2460/ajvr.2005.66.721. Am J Vet Res. 2005. PMID: 15900955
-
Bovine Neutrophils Release Extracellular Traps and Cooperate With Macrophages in Mycobacterium avium subsp. paratuberculosis clearance In Vitro.Front Immunol. 2021 Mar 17;12:645304. doi: 10.3389/fimmu.2021.645304. eCollection 2021. Front Immunol. 2021. PMID: 33815401 Free PMC article.
-
Mycobacterium paratuberculosis and the bovine immune system.Anim Health Res Rev. 2001 Dec;2(2):141-61. Anim Health Res Rev. 2001. PMID: 11831436 Review.
-
The role of IL-10 in Mycobacterium avium subsp. paratuberculosis infection.Cell Commun Signal. 2016 Dec 1;14(1):29. doi: 10.1186/s12964-016-0152-z. Cell Commun Signal. 2016. PMID: 27905994 Free PMC article. Review.
Cited by
-
Immune responses of bison and efficacy after booster vaccination with Brucella abortus strain RB51.Clin Vaccine Immunol. 2015 Apr;22(4):440-7. doi: 10.1128/CVI.00746-14. Epub 2015 Feb 11. Clin Vaccine Immunol. 2015. PMID: 25673305 Free PMC article.
-
Modeling Paratuberculosis in Laboratory Animals, Cells, or Tissues: A Focus on Their Applications for Pathogenesis, Diagnosis, Vaccines, and Therapy Studies.Animals (Basel). 2023 Nov 17;13(22):3553. doi: 10.3390/ani13223553. Animals (Basel). 2023. PMID: 38003170 Free PMC article. Review.
-
Analysis of the Bovine Monocyte-Derived Macrophage Response to Mycobacterium avium Subspecies Paratuberculosis Infection Using RNA-seq.Front Immunol. 2015 Feb 4;6:23. doi: 10.3389/fimmu.2015.00023. eCollection 2015. Front Immunol. 2015. PMID: 25699042 Free PMC article.
-
Synthetic cathelicidin LL-37 reduces Mycobacterium avium subsp. paratuberculosis internalization and pro-inflammatory cytokines in macrophages.Cell Tissue Res. 2020 Jan;379(1):207-217. doi: 10.1007/s00441-019-03098-4. Epub 2019 Sep 2. Cell Tissue Res. 2020. PMID: 31478135 Free PMC article.
-
Identification and Characterization of Mycobacterium smegmatis and Mycobacterium avium subsp. paratuberculosis Zinc Transporters.J Bacteriol. 2021 May 1;203(9):e00049-21. doi: 10.1128/JB.00049-21. Epub 2021 Mar 15. J Bacteriol. 2021. PMID: 33722846 Free PMC article.
References
-
- Ackermann MR, Cheville NF, Deyoe BL. 1988. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Veterinary Pathol. 25:28–35 - PubMed
-
- Alzuherri HM, Woodall CJ, Clarke CJ. 1996. Increased intestinal TNF-alpha, IL-1 beta and IL-6 expression in ovine paratuberculosis. Vet. Immunol. Immunopathol. 49:331–345 - PubMed
-
- Ashida H, et al. 2010. Shigella deploy multiple countermeasures against host innate immune responses. Curr. Opin. Microbiol. - PubMed
-
- Basler T, et al. 2010. TNF- expression in RAW264.7 macrophages infected with pathogenic mycobacteria: evidence for an involvement of lipomannan. J. Leukocyte Biol. 87:173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

