Extracellular Ca(2+) sensing in salivary ductal cells
- PMID: 22778254
- PMCID: PMC3436282
- DOI: 10.1074/jbc.M112.394122
Extracellular Ca(2+) sensing in salivary ductal cells
Abstract
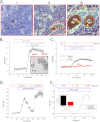
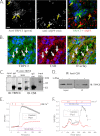
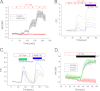
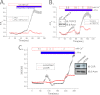
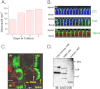
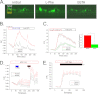
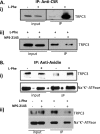
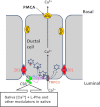
Ca(2+) is secreted from the salivary acinar cells as an ionic constituent of primary saliva. Ions such as Na(+) and Cl(-) get reabsorbed whereas primary saliva flows through the salivary ductal system. Although earlier studies have shown that salivary [Ca(2+)] decreases as it flows down the ductal tree into the oral cavity, ductal reabsorption of Ca(2+) remains enigmatic. Here we report a potential role for the G protein-coupled receptor, calcium-sensing receptor (CSR), in the regulation of Ca(2+) reabsorption by salivary gland ducts. Our data show that CSR is present in the apical region of ductal cells where it is co-localized with transient receptor potential canonical 3 (TRPC3). CSR is activated in isolated salivary gland ducts as well as a ductal cell line (SMIE) by altering extracellular [Ca(2+)] or by aromatic amino acid, L-phenylalanine (L-Phe, endogenous component of saliva), as well as neomycin. CSR activation leads to Ca(2+) influx that, in polarized cells grown on a filter support, is initiated in the luminal region. We show that TRPC3 contributes to Ca(2+) entry triggered by CSR activation. Further, stimulation of CSR in SMIE cells enhances the CSR-TRPC3 association as well as surface expression of TRPC3. Together our findings suggest that CSR could serve as a Ca(2+) sensor in the luminal membrane of salivary gland ducts and regulate reabsorption of [Ca(2+)] from the saliva via TRPC3, thus contributing to maintenance of salivary [Ca(2+)]. CSR could therefore be a potentially important protective mechanism against formation of salivary gland stones (sialolithiasis) and infection (sialoadenitis).
Figures
References
-
- Hoenderop J. G., Nilius B., Bindels R. J. (2005) Calcium absorption across epithelia. Physiol. Rev. 85, 373–422 - PubMed
-
- Knauf H., Lubcke R., Kreutz W., Sachs G. (1982) Interrelationships of ion transport in rat submaxillary duct epithelium. Am. J. Physiol. 242, F132–139 - PubMed
-
- Zentner M. D., Lin H. H., Wen X., Kim K. J., Ann D. K. (1998) The amiloride-sensitive epithelial sodium channel α-subunit is transcriptionally down-regulated in rat parotid cells by the extracellular signal-regulated protein kinase pathway. J. Biol. Chem. 273, 30770–30776 - PubMed
-
- Homann V., Kinne-Saffran E., Arnold W. H., Gaengler P., Kinne R. K. (2006) Calcium transport in human salivary glands: a proposed model of calcium secretion into saliva. Histochem. Cell Biol. 125, 583–591 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

