Toad heart utilizes exclusively slow skeletal muscle troponin T: an evolutionary adaptation with potential functional benefits
- PMID: 22778265
- PMCID: PMC3436204
- DOI: 10.1074/jbc.M112.373191
Toad heart utilizes exclusively slow skeletal muscle troponin T: an evolutionary adaptation with potential functional benefits
Abstract
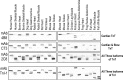
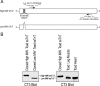
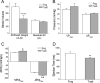
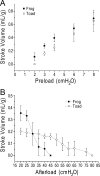
The three isoforms of vertebrate troponin T (TnT) are normally expressed in a muscle type-specific manner. Here we report an exception that the cardiac muscle of toad (Bufo) expresses exclusively slow skeletal muscle TnT (ssTnT) together with cardiac forms of troponin I and myosin as determined using immunoblotting, cDNA cloning, and/or LC-MS/MS. Using RT-PCR and 3'- and 5'-rapid amplification of cDNA ends on toad cardiac mRNA, we cloned full-length cDNAs encoding two alternatively spliced variants of ssTnT. Expression of the cloned cDNAs in Escherichia coli confirmed that the toad cardiac muscle expresses solely ssTnT, predominantly the low molecular weight variant with the exon 5-encoded NH(2)-terminal segment spliced out. Functional studies were performed in ex vivo working toad hearts and compared with the frog (Rana) hearts. The results showed that toad hearts had higher contractile and relaxation velocities and were able to work against a significantly higher afterload than that of frog hearts. Therefore, the unique evolutionary adaptation of utilizing exclusively ssTnT in toad cardiac muscle corresponded to a fitness value from improving systolic function of the heart. The data demonstrated a physiological importance of the functional diversity of TnT isoforms. The structure-function relationship of TnT may be explored for the development of new treatment of heart failure.
Figures





Similar articles
-
TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships.Gene. 2016 May 10;582(1):1-13. doi: 10.1016/j.gene.2016.01.006. Epub 2016 Jan 13. Gene. 2016. PMID: 26774798 Free PMC article. Review.
-
Genomic sequence and structural organization of mouse slow skeletal muscle troponin T gene.Gene. 1999 Mar 18;229(1-2):1-10. doi: 10.1016/s0378-1119(99)00051-7. Gene. 1999. PMID: 10095098
-
Slow troponin T mRNA in striated muscles is expressed in both cell type and developmental stage specific manner.J Muscle Res Cell Motil. 2000;21(6):527-36. doi: 10.1023/a:1026541803317. J Muscle Res Cell Motil. 2000. PMID: 11206131
-
Three alternatively spliced mouse slow skeletal muscle troponin T isoforms: conserved primary structure and regulated expression during postnatal development.Gene. 1998 Jul 3;214(1-2):121-9. doi: 10.1016/s0378-1119(98)00214-5. Gene. 1998. PMID: 9651500
-
Troponin T isoforms and posttranscriptional modifications: Evolution, regulation and function.Arch Biochem Biophys. 2011 Jan 15;505(2):144-54. doi: 10.1016/j.abb.2010.10.013. Epub 2010 Oct 18. Arch Biochem Biophys. 2011. PMID: 20965144 Free PMC article. Review.
Cited by
-
Role of the interaction between troponin T and AMP deaminase by zinc bridge in modulating muscle contraction and ammonia production.Mol Cell Biochem. 2024 Apr;479(4):793-809. doi: 10.1007/s11010-023-04763-7. Epub 2023 May 15. Mol Cell Biochem. 2024. PMID: 37184757 Free PMC article. Review.
-
Evolution of the N-Terminal Regulation of Cardiac Troponin I for Heart Function of Tetrapods: Lungfish Presents an Example of the Emergence of Novel Submolecular Structure to Lead the Capacity of Adaptation.J Mol Evol. 2022 Feb;90(1):30-43. doi: 10.1007/s00239-021-10039-9. Epub 2021 Dec 29. J Mol Evol. 2022. PMID: 34966949 Free PMC article.
-
TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships.Gene. 2016 May 10;582(1):1-13. doi: 10.1016/j.gene.2016.01.006. Epub 2016 Jan 13. Gene. 2016. PMID: 26774798 Free PMC article. Review.
-
Identification and Characterization of Troponin T Associated with Development, Metabolism and Reproduction in Tribolium castaneum.Int J Mol Sci. 2025 Mar 19;26(6):2786. doi: 10.3390/ijms26062786. Int J Mol Sci. 2025. PMID: 40141428 Free PMC article.
-
Cardiac troponin T and fast skeletal muscle denervation in ageing.J Cachexia Sarcopenia Muscle. 2017 Oct;8(5):808-823. doi: 10.1002/jcsm.12204. Epub 2017 Apr 16. J Cachexia Sarcopenia Muscle. 2017. PMID: 28419739 Free PMC article.
References
-
- Boral M. C., Deb C. (1970) Seasonal changes in body fluids and hematology in toad (Bufo melanostictus)—a poikilothermic cold torpor. Proc. Indian Natl. Sci. Acad. 36, 369–373
-
- Deb C., Chatterjee S., Boral M. C. (1974) Body fluid and hematological changes in toads following heat exposure. Am. J. Physiol. 226, 408–410 - PubMed
-
- Schadt J. C., Ludbrook J. (1991) Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am. J. Physiol. 260, H305-H318 - PubMed
-
- Peitzman A. B., Billiar T. R., Harbrecht B. G., Kelly E., Udekwu A. O., Simmons R. L. (1995) Hemorrhagic shock. Curr. Probl. Surg. 32, 925–1002 - PubMed
-
- Gordon A. M., Homsher E., Regnier M. (2000) Regulation of contraction in striated muscle. Physiol. Rev. 80, 853–924 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

