Use of quantitative membrane proteomics identifies a novel role of mitochondria in healing injured muscles
- PMID: 22778268
- PMCID: PMC3436295
- DOI: 10.1074/jbc.M112.354415
Use of quantitative membrane proteomics identifies a novel role of mitochondria in healing injured muscles
Abstract
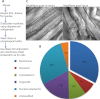
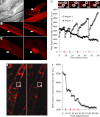
Skeletal muscles are proficient at healing from a variety of injuries. Healing occurs in two phases, early and late phase. Early phase involves healing the injured sarcolemma and restricting the spread of damage to the injured myofiber. Late phase of healing occurs a few days postinjury and involves interaction of injured myofibers with regenerative and inflammatory cells. Of the two phases, cellular and molecular processes involved in the early phase of healing are poorly understood. We have implemented an improved sarcolemmal proteomics approach together with in vivo labeling of proteins with modified amino acids in mice to study acute changes in the sarcolemmal proteome in early phase of myofiber injury. We find that a notable early phase response to muscle injury is an increased association of mitochondria with the injured sarcolemma. Real-time imaging of live myofibers during injury demonstrated that the increased association of mitochondria with the injured sarcolemma involves translocation of mitochondria to the site of injury, a response that is lacking in cultured myoblasts. Inhibiting mitochondrial function at the time of injury inhibited healing of the injured myofibers. This identifies a novel role of mitochondria in the early phase of healing injured myofibers.
Figures



References
-
- Bansal D., Miyake K., Vogel S. S., Groh S., Chen C. C., Williamson R., McNeil P. L., Campbell K. P. (2003) Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423, 168–172 - PubMed
-
- Carpenter S., Karpati G. (1989) Segmental necrosis and its demarcation in experimental micropuncture injury of skeletal muscle fibers. J. Neuropathol. Exp. Neurol. 48, 154–170 - PubMed
-
- Papadimitriou J. M., Robertson T. A., Mitchell C. A., Grounds M. D. (1990) The process of new plasmalemma formation in focally injured skeletal muscle fibers. J. Struct. Biol. 103, 124–134 - PubMed
-
- Faulkner J. A., Brooks S. V., Opiteck J. A. (1993) Injury to skeletal muscle fibers during contractions. Conditions of occurrence and prevention. Phys. Ther. 73, 911–921 - PubMed
-
- Grounds M. D. (1991) Toward understanding skeletal muscle regeneration. Pathol. Res. Pract. 187, 1–22 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

