Thioredoxin reductase linked to cytoskeleton by focal adhesion kinase reverses actin S-nitrosylation and restores neutrophil β(2) integrin function
- PMID: 22778269
- PMCID: PMC3436286
- DOI: 10.1074/jbc.M112.355875
Thioredoxin reductase linked to cytoskeleton by focal adhesion kinase reverses actin S-nitrosylation and restores neutrophil β(2) integrin function
Abstract
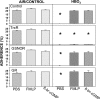
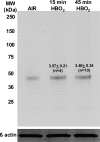
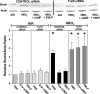
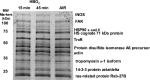
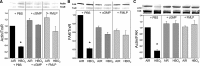
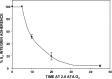
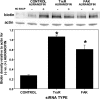
The investigation goal was to identify mechanisms for reversal of actin S-nitrosylation in neutrophils after exposure to high oxygen partial pressures. Prior work has shown that hyperoxia causes S-nitrosylated actin (SNO-actin) formation, which mediates β(2) integrin dysfunction, and these changes can be reversed by formylmethionylleucylphenylalanine or 8-bromo-cyclic GMP. Herein we show that thioredoxin reductase (TrxR) is responsible for actin denitrosylation. Approximately 80% of cellular TrxR is localized to the cytosol, divided between the G-actin and short filamentous actin (sF-actin) fractions based on Triton solubility of cell lysates. TrxR linkage to sF-actin requires focal adhesion kinase (FAK) based on immunoprecipitation studies. S-Nitrosylation accelerates actin filament turnover (by mechanisms described previously (Thom, S. R., Bhopale, V. M., Yang, M., Bogush, M., Huang, S., and Milovanova, T. (2011) Neutrophil β(2) integrin inhibition by enhanced interactions of vasodilator stimulated phosphoprotein with S-nitrosylated actin. J. Biol. Chem. 286, 32854-32865), which causes FAK to disassociate from sF-actin. TrxR subsequently dissociates from FAK, and the physical separation from actin impedes denitrosylation. If SNO-actin is photochemically reduced with UV light or if actin filament turnover is impeded by incubations with cytochalasin D, latrunculin B, 8-bromo-cGMP, or formylmethionylleucylphenylalanine, FAK and TrxR reassociate with sF-actin and cause SNO-actin removal. FAK-TrxR association can also be demonstrated using isolated enzymes in ex vivo preparations. Uniquely, the FAK kinase domain is the site of TrxR linkage. We conclude that through its scaffold function, FAK influences TrxR activity and actin S-nitrosylation.
Figures



References
-
- Brown E. J., Lindberg F. P. (1996) Leukocyte adhesion molecules in host defence against infection. Ann. Med. 28, 201–208 - PubMed
-
- Thom S. R. (1993) Functional inhibition of leukocyte B2 integrins by hyperbaric oxygen in carbon monoxide-mediated brain injury in rats. Toxicol. Appl. Pharmacol. 123, 248–256 - PubMed
-
- Thom S. R., Mendiguren I., Hardy K., Bolotin T., Fisher D., Nebolon M., Kilpatrick L. (1997) Inhibition of human neutrophil β2-integrin-dependent adherence by hyperbaric O2. Am. J. Physiol. 272, C770–C777 - PubMed
-
- Kalns J., Lane J., Delgado A., Scruggs J., Ayala E., Gutierrez E., Warren D., Niemeyer D., George Wolf E., Bowden R. A. (2002) Hyperbaric oxygen exposure temporarily reduces Mac-1-mediated functions of human neutrophils. Immunol. Lett. 83, 125–131 - PubMed
-
- Labrouche S., Javorschi S., Leroy D., Gbikpi-Benissan G., Freyburger G. (1999) Influence of hyperbaric oxygen on leukocyte functions and hemostasis in normal volunteer divers. Thromb. Res. 96, 309–315 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

