Thymocytes may persist and differentiate without any input from bone marrow progenitors
- PMID: 22778388
- PMCID: PMC3420331
- DOI: 10.1084/jem.20120845
Thymocytes may persist and differentiate without any input from bone marrow progenitors
Abstract
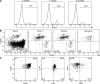
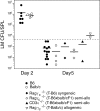
Thymus transplants can correct deficiencies of the thymus epithelium caused by the complete DiGeorge syndrome or FOXN1 mutations. However, thymus transplants were never used to correct T cell-intrinsic deficiencies because it is generally believed that thymocytes have short intrinsic lifespans. This notion is based on thymus transplantation experiments where it was shown that thymus-resident cells were rapidly replaced by progenitors originating in the bone marrow. In contrast, here we show that neonatal thymi transplanted into interleukin 7 receptor-deficient hosts harbor populations with extensive capacity to self-renew, and maintain continuous thymocyte generation and export. These thymus transplants reconstitute the full diversity of peripheral T cell repertoires one month after surgery, which is the earliest time point studied. Moreover, transplantation experiments performed across major histocompatibility barriers show that allogeneic transplanted thymi are not rejected, and allogeneic cells do not induce graft-versus-host disease; transplants induced partial or total protection to infection. These results challenge the current dogma that thymocytes cannot self-renew, and indicate a potential use of neonatal thymus transplants to correct T cell-intrinsic deficiencies. Finally, as found with mature T cells, they show that thymocyte survival is determined by the competition between incoming progenitors and resident cells.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

