GPR105 ablation prevents inflammation and improves insulin sensitivity in mice with diet-induced obesity
- PMID: 22778393
- PMCID: PMC3411902
- DOI: 10.4049/jimmunol.1103207
GPR105 ablation prevents inflammation and improves insulin sensitivity in mice with diet-induced obesity
Erratum in
- J Immunol. 2013 Feb 1;190(3):1380. Mamane, Yaël [added]; Mancini, Joseph A [added]; Nawrocki, Andrea R [added]
Abstract
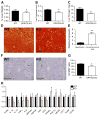
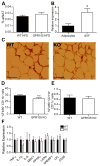
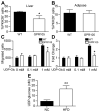
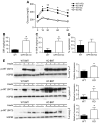
GPR105, a G protein-coupled receptor for UDP-glucose, is highly expressed in several human tissues and participates in the innate immune response. Because inflammation has been implicated as a key initial trigger for type 2 diabetes, we hypothesized that GPR105 (official gene name: P2RY14) might play a role in the initiation of inflammation and insulin resistance in obesity. To this end, we investigated glucose metabolism in GPR105 knockout (KO) and wild-type (WT) mice fed a high-fat diet (HFD). We also examined whether GPR105 regulates macrophage recruitment to liver or adipose tissues by in vivo monocyte tracking and in vitro chemotaxis experiments, followed by transplantation of bone marrow from either KO or WT donors to WT recipients. Our data show that genetic deletion of GPR105 confers protection against HFD-induced insulin resistance, with reduced macrophage infiltration and inflammation in liver, and increased insulin-stimulated Akt phosphorylation in liver, muscle, and adipose tissue. By tracking monocytes from either KO or WT donors, we found that fewer KO monocytes were recruited to the liver of WT recipients. Furthermore, we observed that uridine 5-diphosphoglucose enhanced the in vitro migration of bone marrow-derived macrophages from WT but not KO mice, and that plasma uridine 5-diphosphoglucose levels were significantly higher in obese versus lean mice. Finally, we confirmed that insulin sensitivity improved in HFD mice with a myeloid cell-specific deletion of GPR105. These studies indicate that GPR105 ablation mitigates HFD-induced insulin resistance by inhibiting macrophage recruitment and tissue inflammation. Hence GPR105 provides a novel link between innate immunity and metabolism.
Figures


References
-
- Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annual Review of Physiology. 2010;72:219–246. - PubMed
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. - PubMed
-
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. - PubMed
-
- Thewissen MM, Damoiseaux JG, Duijvestijn AM, van Greevenbroek MM, van der Kallen CJ, Feskens EJ, Blaak EE, Schalkwijk CG, Stehouwer CD, Cohen Tervaert JW, Ferreira I. Abdominal fat mass is associated with adaptive immune activation: the CODAM Study. Obesity (Silver Spring) 2011;19:1690–1698. - PubMed
-
- Filmore D. Modern Drug Discovery. American Chemical Society; 2004. It’s a GPCR world; pp. 24–28.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

