Chemotactic signaling via carbohydrate phosphotransferase systems in Escherichia coli
- PMID: 22778402
- PMCID: PMC3409764
- DOI: 10.1073/pnas.1205307109
Chemotactic signaling via carbohydrate phosphotransferase systems in Escherichia coli
Abstract
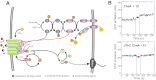
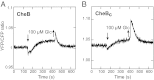
Chemotaxis allows bacteria to follow gradients of nutrients, environmental stimuli, and signaling molecules, optimizing bacterial growth and survival. Escherichia coli has long served as a model of bacterial chemotaxis, and the signal processing by the core of its chemotaxis pathway is well understood. However, most of the research so far has focused on one branch of chemotactic signaling, in which ligands bind to periplasmic sensory domains of transmembrane chemoreceptors and induce a conformational change that is transduced across the membrane to regulate activity of the receptor-associated kinase CheA. Here we quantitatively characterize another, receptor-independent branch of chemotactic signaling that is linked to the sugar uptake through a large family of phosphotransferase systems (PTSs). Using in vivo characterization of intracellular signaling and protein interactions, we demonstrate that signals from cytoplasmic PTS components are transmitted directly to the sensory complexes formed by chemoreceptors, CheA and an adapter protein CheW. We further conclude that despite different modes of sensing, the PTS- and receptor-mediated signals have similar regulatory effects on the conformation of the sensory complexes. As a consequence, both types of signals become integrated and undergo common downstream processing including methylation-dependent adaptation. We propose that such mode of signaling is essential for efficient chemotaxis to PTS substrates and may be common to most bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Coupling the phosphotransferase system and the methyl-accepting chemotaxis protein-dependent chemotaxis signaling pathways of Escherichia coli.Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11583-7. doi: 10.1073/pnas.92.25.11583. Proc Natl Acad Sci U S A. 1995. PMID: 8524808 Free PMC article.
-
Elucidation of a PTS-carbohydrate chemotactic signal pathway in Escherichia coli using a time-resolved behavioral assay.Mol Biol Cell. 1999 Apr;10(4):1133-46. doi: 10.1091/mbc.10.4.1133. Mol Biol Cell. 1999. PMID: 10198062 Free PMC article.
-
Core unit of chemotaxis signaling complexes.Proc Natl Acad Sci U S A. 2011 Jun 7;108(23):9390-5. doi: 10.1073/pnas.1104824108. Epub 2011 May 23. Proc Natl Acad Sci U S A. 2011. PMID: 21606342 Free PMC article.
-
Bacterial chemotaxis coupling protein: Structure, function and diversity.Microbiol Res. 2019 Feb;219:40-48. doi: 10.1016/j.micres.2018.11.001. Epub 2018 Nov 6. Microbiol Res. 2019. PMID: 30642465 Review.
-
Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update.Trends Microbiol. 2015 May;23(5):257-66. doi: 10.1016/j.tim.2015.03.003. Epub 2015 Mar 30. Trends Microbiol. 2015. PMID: 25834953 Free PMC article. Review.
Cited by
-
Endophytism: A Multidimensional Approach to Plant-Prokaryotic Microbe Interaction.Front Microbiol. 2022 May 12;13:861235. doi: 10.3389/fmicb.2022.861235. eCollection 2022. Front Microbiol. 2022. PMID: 35633681 Free PMC article. Review.
-
The dCache Domain of the Chemoreceptor Tlp1 in Campylobacter jejuni Binds and Triggers Chemotaxis toward Formate.mBio. 2023 Jun 27;14(3):e0356422. doi: 10.1128/mbio.03564-22. Epub 2023 Apr 13. mBio. 2023. PMID: 37052512 Free PMC article.
-
A cheZ-Like Gene in Azorhizobium caulinodans Is a Key Gene in the Control of Chemotaxis and Colonization of the Host Plant.Appl Environ Microbiol. 2018 Jan 17;84(3):e01827-17. doi: 10.1128/AEM.01827-17. Print 2018 Feb 1. Appl Environ Microbiol. 2018. PMID: 29150498 Free PMC article.
-
Variation of swimming speed enhances the chemotactic migration of Escherichia coli.Syst Synth Biol. 2015 Sep;9(3):85-95. doi: 10.1007/s11693-015-9174-x. Epub 2015 Jul 9. Syst Synth Biol. 2015. PMID: 26279703 Free PMC article.
-
Glucose Transport in Escherichia coli: From Basics to Transport Engineering.Microorganisms. 2023 Jun 15;11(6):1588. doi: 10.3390/microorganisms11061588. Microorganisms. 2023. PMID: 37375089 Free PMC article. Review.
References
-
- Alon U, Surette MG, Barkai N, Leibler S. Robustness in bacterial chemotaxis. Nature. 1999;397:168–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

