Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity
- PMID: 22778410
- PMCID: PMC3409774
- DOI: 10.1073/pnas.1202835109
Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity
Abstract
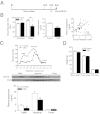
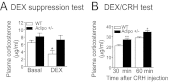
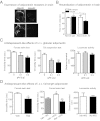
Depression is a debilitating mental illness and is often comorbid with metabolic disorders such as type 2 diabetes. Adiponectin is an adipocyte-derived hormone with antidiabetic and insulin-sensitizing properties. Here we show that adiponectin levels in plasma are reduced in a chronic social-defeat stress model of depression, which correlates with decreased social interaction time. A reduction in adiponectin levels caused by haploinsufficiency results in increased susceptibility to social aversion, "anhedonia," and learned helplessness and causes impaired glucocorticoid-mediated negative feedback on the hypothalamic-pituitary-adrenal (HPA) axis. Intracerebroventricular (i.c.v.) injection of an adiponectin neutralizing antibody precipitates stress-induced depressive-like behavior. Conversely, i.c.v. administration of exogenous adiponectin produces antidepressant-like behavioral effects in normal-weight mice and in diet-induced obese diabetic mice. Taken together, these results suggest a critical role of adiponectin in depressive-like behaviors and point to a potential innovative therapeutic approach for depressive disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Neurogenesis-independent antidepressant-like effects of enriched environment is dependent on adiponectin.Psychoneuroendocrinology. 2015 Jul;57:72-83. doi: 10.1016/j.psyneuen.2015.03.017. Epub 2015 Apr 9. Psychoneuroendocrinology. 2015. PMID: 25889841
-
Ketamine improved depressive-like behaviors via hippocampal glucocorticoid receptor in chronic stress induced- susceptible mice.Behav Brain Res. 2019 May 17;364:75-84. doi: 10.1016/j.bbr.2019.01.057. Epub 2019 Feb 10. Behav Brain Res. 2019. PMID: 30753876
-
Bifidobacterium pseudocatenulatum CECT 7765 Ameliorates Neuroendocrine Alterations Associated with an Exaggerated Stress Response and Anhedonia in Obese Mice.Mol Neurobiol. 2018 Jun;55(6):5337-5352. doi: 10.1007/s12035-017-0768-z. Epub 2017 Sep 18. Mol Neurobiol. 2018. PMID: 28921462
-
Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression.Neuroscience. 2011 Sep 29;192:661-74. doi: 10.1016/j.neuroscience.2011.05.075. Epub 2011 Jun 15. Neuroscience. 2011. PMID: 21712072
-
Antidepressant-like effects of Xiaochaihutang in a neuroendocrine mouse model of anxiety/depression.J Ethnopharmacol. 2016 Dec 24;194:674-683. doi: 10.1016/j.jep.2016.10.028. Epub 2016 Oct 13. J Ethnopharmacol. 2016. PMID: 27746334
Cited by
-
A change in objective sleep duration is associated with a change in the serum adiponectin level of women with overweight or obesity undergoing weight loss intervention.Obes Sci Pract. 2016 Jun;2(2):180-188. doi: 10.1002/osp4.32. Epub 2016 Mar 14. Obes Sci Pract. 2016. PMID: 27812383 Free PMC article.
-
Impact of fasting on stress systems and depressive symptoms in patients with major depressive disorder: a cross-sectional study.Sci Rep. 2022 May 10;12(1):7642. doi: 10.1038/s41598-022-11639-1. Sci Rep. 2022. PMID: 35538177 Free PMC article.
-
Role of Adiponectin in Central Nervous System Disorders.Neural Plast. 2018 Jul 29;2018:4593530. doi: 10.1155/2018/4593530. eCollection 2018. Neural Plast. 2018. PMID: 30150999 Free PMC article. Review.
-
Role and mechanism of PVN-sympathetic-adipose circuit in depression and insulin resistance induced by chronic stress.EMBO Rep. 2023 Dec 6;24(12):e57176. doi: 10.15252/embr.202357176. Epub 2023 Oct 23. EMBO Rep. 2023. PMID: 37870400 Free PMC article.
-
Adiponectin and depression: A meta-analysis.Biomed Rep. 2015 Jan;3(1):38-42. doi: 10.3892/br.2014.372. Epub 2014 Oct 16. Biomed Rep. 2015. PMID: 25469244 Free PMC article.
References
-
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. - PubMed
-
- Knol MJ, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–845. - PubMed
-
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–1078. - PubMed
-
- Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166:591–598. - PubMed
-
- Brown LC, Majumdar SR, Johnson JA. Type of antidepressant therapy and risk of type 2 diabetes in people with depression. Diabetes Res Clin Pract. 2008;79:61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

