Effects of peptides derived from terminal modifications of the aβ central hydrophobic core on aβ fibrillization
- PMID: 22778807
- PMCID: PMC3368634
- DOI: 10.1021/cn900019r
Effects of peptides derived from terminal modifications of the aβ central hydrophobic core on aβ fibrillization
Abstract
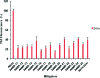
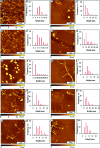
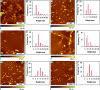
Considerable research effort has focused on the discovery of mitigators that block the toxicity of the β-amyloid peptide (Aβ) by targeting a specific step involved in Aβ fibrillogenesis and subsequent aggregation. Given that aggregation intermediates are hypothesized to be responsible for Aβ toxicity, such compounds could likely prevent or mitigate aggregation, or alternatively cause further association of toxic oligomers into larger nontoxic aggregates. Herein we investigate the effect of modifications of the KLVFF hydrophobic core of Aβ by replacing N- and C-terminal groups with various polar moieties. Several of these terminal modifications were found to disrupt the formation of amyloid fibrils and in some cases induced the disassembly of preformed fibrils. Significantly, mitigators that incorporate MiniPEG polar groups were found to be effective against Aβ(1-40) fibrilligonesis. Previously, we have shown that mitigators incorporating alpha,alpha-disubstituted amino acids (ααAAs) were effective in disrupting fibril formation as well as inducing fibril disassembly. In this work, we further disclose that the number of polar residues (six) and ααAAs (three) in the original mitigator can be reduced without dramatically changing the ability to disrupt Aβ(1-40) fibrillization in vitro.
Keywords: Amyloid peptide (Aβ); assembly; disassembly; fibrils; mitigators; spherical structures.
Figures


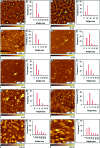
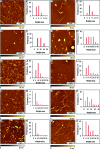
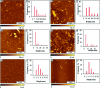
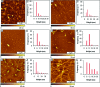
References
-
- Selkoe D. J. (1991) The molecular pathology of Alzheimers-disease. Neuron 6, 487–498. - PubMed
-
- Selkoe D. J. (2003) Folding proteins in fatal ways. Nature 426, 900–904. - PubMed
-
- Tycko R. (2003) Insights into the amyloid folding problem from solid-state NMR. Biochemistry 42, 3151–3159. - PubMed
-
- Chromy B. A.; Nowak R. J.; Lambert M. P.; Viola K. L.; Chang L.; Velasco P. T.; Jones B. W.; Fernandez S. J.; Lacor P. N.; Horowitz P.; Finch C. E.; Krafft G. A.; Klein W. L. (2003) Self-assembly of A beta(1−42) into globular neurotoxins. Biochemistry 42, 12749–12760. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

