In vivo characterization of a smart MRI agent that displays an inverse response to calcium concentration
- PMID: 22778817
- PMCID: PMC3368642
- DOI: 10.1021/cn100083a
In vivo characterization of a smart MRI agent that displays an inverse response to calcium concentration
Abstract
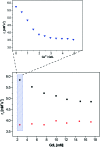
Contrast agents for magnetic resonance imaging (MRI) that exhibit sensitivity toward specific ions or molecules represent a challenging but attractive direction of research. Here a Gd(3+) complex linked to an aminobis(methylenephosphonate) group for chelating Ca(2+) was synthesized and investigated. The longitudinal relaxivity (r(1)) of this complex decreases during the relaxometric titration with Ca(2+) from 5.76 to 3.57 mM(-1) s(-1) upon saturation. The r(1) is modulated by changes in the hydration number, which was confirmed by determination of the luminescence emission lifetimes of the analogous Eu(3+) complex. The initial in vivo characterization of this responsive contrast agent was performed by means of electrophysiology and MRI experiments. The investigated complex is fully biocompatible, having no observable effect on neuronal function after administration into the brain ventricles or parenchyma. Distribution studies demonstrated that the diffusivity of this agent is significantly lower compared with that of gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA).
Keywords: Smart contrast agents; calcium signaling; functional magnetic resonance imaging (fMRI); in vivo brain imaging.
Figures





Similar articles
-
Impact of Treatment With Chelating Agents Depends on the Stability of Administered GBCAs: A Comparative Study in Rats.Invest Radiol. 2019 Feb;54(2):76-82. doi: 10.1097/RLI.0000000000000522. Invest Radiol. 2019. PMID: 30358694 Free PMC article.
-
Facile synthesis and relaxation properties of novel bispolyazamacrocyclic Gd3+ complexes: an attempt towards calcium-sensitive MRI contrast agents.Inorg Chem. 2008 Feb 18;47(4):1370-81. doi: 10.1021/ic7017456. Epub 2008 Jan 1. Inorg Chem. 2008. PMID: 18166011
-
Influence of calcium-induced aggregation on the sensitivity of aminobis(methylenephosphonate)-containing potential MRI contrast agents.Inorg Chem. 2011 Jul 18;50(14):6472-81. doi: 10.1021/ic1024235. Epub 2011 Jun 14. Inorg Chem. 2011. PMID: 21671565
-
Paramagnetic water-soluble metallofullerenes having the highest relaxivity for MRI contrast agents.Bioconjug Chem. 2001 Jul-Aug;12(4):510-4. doi: 10.1021/bc000136m. Bioconjug Chem. 2001. PMID: 11459454
-
Distribution and chemical forms of gadolinium in the brain: a review.Br J Radiol. 2017 Nov;90(1079):20170115. doi: 10.1259/bjr.20170115. Epub 2017 Sep 28. Br J Radiol. 2017. PMID: 28749164 Free PMC article. Review.
Cited by
-
Calcium-responsive contrast agents for functional magnetic resonance imaging.Chem Phys Rev. 2021 Jun;2(2):021301. doi: 10.1063/5.0041394. Chem Phys Rev. 2021. PMID: 34085055 Free PMC article. Review.
-
Single (19)F probe for simultaneous detection of multiple metal ions using miCEST MRI.J Am Chem Soc. 2015 Jan 14;137(1):78-81. doi: 10.1021/ja511313k. Epub 2014 Dec 22. J Am Chem Soc. 2015. PMID: 25523816 Free PMC article.
-
A Protein-Based Biosensor for Detecting Calcium by Magnetic Resonance Imaging.ACS Sens. 2021 Sep 24;6(9):3163-3169. doi: 10.1021/acssensors.1c01085. Epub 2021 Aug 22. ACS Sens. 2021. PMID: 34420291 Free PMC article.
-
Investigation of a calcium-responsive contrast agent in cellular model systems: feasibility for use as a smart molecular probe in functional MRI.ACS Chem Neurosci. 2014 May 21;5(5):360-9. doi: 10.1021/cn500049n. Epub 2014 Apr 8. ACS Chem Neurosci. 2014. PMID: 24712900 Free PMC article.
-
Advanced optoacoustic methods for multiscale imaging of in vivo dynamics.Chem Soc Rev. 2017 Apr 18;46(8):2158-2198. doi: 10.1039/c6cs00765a. Chem Soc Rev. 2017. PMID: 28276544 Free PMC article. Review.
References
-
- Kandel E. R., Schwartz J. H., Jessell T. M. (2000) Principles of Neural Science, 4th ed., McGraw-Hill, New York; London.
-
- Merbach A. E., Toth E. (2001) The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, Wiley, Chichester, U.K.
-
- Caravan P.; Ellison J. J.; McMurry T. J.; Lauffer R. B. (1999) Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 99, 2293–2352. - PubMed
-
- Que E. L.; Chang C. J. (2010) Responsive magnetic resonance imaging contrast agents as chemical sensors for metals in biology and medicine. Chem. Soc. Rev. 39, 51–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

