Improved neuronal tract tracing with stable biocytin-derived neuroimaging agents
- PMID: 22778821
- PMCID: PMC3368650
- DOI: 10.1021/cn900010d
Improved neuronal tract tracing with stable biocytin-derived neuroimaging agents
Abstract
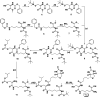
One of the main characteristics of brains is their profuse connectivity at different spatial scales. Understanding brain function evidently first requires a comprehensive description of neuronal anatomical connections. Not surprisingly a large number of histological markers were developed over the years that can be used for tracing mono- or polysynaptic connections. Biocytin is a classical neuroanatomical tracer commonly used to map brain connectivity. However, the endogenous degradation of the molecule by the action of biotinidase enzymes precludes its applicability in long-term experiments and limits the quality and completeness of the rendered connections. With the aim to improve the stability of this classical tracer, two novel biocytin-derived compounds were designed and synthesized. Here we present their greatly improved stability in biological tissue along with retained capacity to function as neuronal tracers. The experiments, 24 and 96 h postinjection, demonstrated that the newly synthesized molecules yielded more detailed and complete information about brain networks than that obtained with conventional biocytin. Preliminary results suggest that the reported molecular designs can be further diversified for use as multimodal tracers in combined MRI and optical or electron microscopy experiments.
Keywords: Brain mapping; biocytin; biotinidase; histology; neuroanatomical tracers.
Figures






Similar articles
-
Biocytin-derived MRI contrast agent for longitudinal brain connectivity studies.ACS Chem Neurosci. 2011 Oct 19;2(10):578-87. doi: 10.1021/cn200022m. Epub 2011 Aug 3. ACS Chem Neurosci. 2011. PMID: 22860157 Free PMC article.
-
A method of combining biocytin tract-tracing with avidin-biotin-peroxidase complex immunocytochemistry for pre-embedding electron microscopic labeling in neonatal tissue.J Neurosci Methods. 1998 Jun 1;81(1-2):189-97. doi: 10.1016/s0165-0270(98)00039-9. J Neurosci Methods. 1998. PMID: 9696325
-
In vitro biocytin injection into perinatal mouse brain: a method for tract tracing in developing tissue.J Neurosci Methods. 2000 Apr 1;97(1):1-6. doi: 10.1016/s0165-0270(99)00190-9. J Neurosci Methods. 2000. PMID: 10771069
-
Neuroanatomical labeling with biocytin: a review.Neuroreport. 1992 Oct;3(10):821-7. doi: 10.1097/00001756-199210000-00001. Neuroreport. 1992. PMID: 1384763 Review.
-
Neural convergence and divergence in the mammalian cerebral cortex: from experimental neuroanatomy to functional neuroimaging.J Comp Neurol. 2013 Dec 15;521(18):4097-111. doi: 10.1002/cne.23408. J Comp Neurol. 2013. PMID: 23840023 Free PMC article. Review.
Cited by
-
Synthesis and in vitro evaluation of a biotinylated dextran-derived probe for molecular imaging.ACS Chem Neurosci. 2012 Apr 18;3(4):268-73. doi: 10.1021/cn200112v. Epub 2012 Jan 16. ACS Chem Neurosci. 2012. PMID: 22860193 Free PMC article.
-
Biocytin-Labeling in Whole-Cell Recording: Electrophysiological and Morphological Properties of Pyramidal Neurons in CYLD-Deficient Mice.Molecules. 2023 May 15;28(10):4092. doi: 10.3390/molecules28104092. Molecules. 2023. PMID: 37241833 Free PMC article.
-
Fragile X Mental Retardation Protein Restricts Small Dye Iontophoresis Entry into Central Neurons.J Neurosci. 2017 Oct 11;37(41):9844-9858. doi: 10.1523/JNEUROSCI.0723-17.2017. Epub 2017 Sep 8. J Neurosci. 2017. PMID: 28887386 Free PMC article.
References
-
- King M. A.; Louis P. M.; Hunter B. E.; Walker D. W. (1989) Biocytin: A versatile anterograde neuroanatomical tract-tracing alternative. Brain Res. 497(2), 361–367. - PubMed
-
- Lapper S. R.; Bolam J. P. (1991) The anterograde and retrograde transport of neurobiotin in the central nervous system of the rat: Comparison with biocytin. J. Neurosci. Methods 39(2), 163–174. - PubMed
-
- Kobbert C.; Apps R.; Bechmann I.; Lanciego J. L.; Mey J.; Thanos S. (2000) Current concepts in neuroanatomical tracing. Prog. Neurobiol. 62(4), 327–351. - PubMed
-
- Izzo P. N. (1991) A note on the use of biocytin in anterograde tracing studies in the central nervous system: Application at both light and electron microscopic level. J. Neurosci. Methods 36(2−3), 155–166. - PubMed
-
- Horikawa K.; Armstrong W. E. (1988) A versatile means of intracellular labeling: Injection of biocytin and its detection with avidin conjugates. J. Neurosci. Methods 25(1), 1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

