Quantum dot labeling of butyrylcholinesterase maintains substrate and inhibitor interactions and cell adherence features
- PMID: 22778863
- PMCID: PMC3369731
- DOI: 10.1021/cn1000827
Quantum dot labeling of butyrylcholinesterase maintains substrate and inhibitor interactions and cell adherence features
Abstract
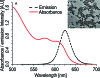
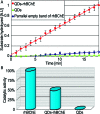
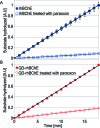
Butyrylcholinesterase (BChE) is the major acetylcholine hydrolyzing enzyme in peripheral mammalian systems. It can either reside in the circulation or adhere to cells and tissues and protect them from anticholinesterases, including insecticides and poisonous nerve gases. In humans, impaired cholinesterase functioning is causally involved in many pathologies, including Alzheimer's and Parkinson's diseases, trait anxiety, and post stroke conditions. Recombinant cholinesterases have been developed for therapeutic use; therefore, it is important to follow their in vivo path, location, and interactions. Traditional labeling methods, such as fluorescent dyes and proteins, generally suffer from sensitivity to environmental conditions, from proximity to different molecules or special enzymes which can alter them, and from relatively fast photobleaching. In contrast, emerging development in synthesis and surface engineering of semiconductor nanocrystals enable their use to detect and follow molecules in biological milieus at high sensitivity and in real time. Therefore, we developed a platform for conjugating highly purified recombinant human BChE dimers (rhBChE) to CdSe/CdZnS quantum dots (QDs). We report the development and characterization of highly fluorescent aqueous soluble QD-rhBChE conjugates, present maintenance of hydrolytic activity, inhibitor sensitivity, and adherence to the membrane of cultured live cells of these conjugates, and outline their advantageous features for diverse biological applications.
Keywords: Anticholinesterases; bioconjugation; butyrylcholinesterase; confocal microscopy; quantum dots; transmission electron microscopy.
Figures




Similar articles
-
Plant-expressed cocaine hydrolase variants of butyrylcholinesterase exhibit altered allosteric effects of cholinesterase activity and increased inhibitor sensitivity.Sci Rep. 2017 Sep 5;7(1):10419. doi: 10.1038/s41598-017-10571-z. Sci Rep. 2017. PMID: 28874829 Free PMC article.
-
Quantum dots-based fluorescent probes for turn-on and turn-off sensing of butyrylcholinesterase.Biosens Bioelectron. 2013 Jun 15;44:204-9. doi: 10.1016/j.bios.2013.01.034. Epub 2013 Jan 29. Biosens Bioelectron. 2013. PMID: 23428734
-
Polyionic complexes of butyrylcholinesterase and poly-l-lysine-g-poly(ethylene glycol): Comparative kinetics of catalysis and inhibition and in vitro inactivation by proteases and heat.Chem Biol Interact. 2017 Sep 25;275:86-94. doi: 10.1016/j.cbi.2017.07.019. Epub 2017 Jul 27. Chem Biol Interact. 2017. PMID: 28756151
-
The origin of the molecular diversity and functional anchoring of cholinesterases.Neurosignals. 2002 May-Jun;11(3):130-43. doi: 10.1159/000065054. Neurosignals. 2002. PMID: 12138250 Review.
-
Quantum dot surface chemistry and functionalization for cell targeting and imaging.Bioconjug Chem. 2015 Apr 15;26(4):609-24. doi: 10.1021/acs.bioconjchem.5b00069. Epub 2015 Mar 13. Bioconjug Chem. 2015. PMID: 25710410 Review.
Cited by
-
Cholinesterases and the fine line between poison and remedy.Biochem Pharmacol. 2018 Jul;153:205-216. doi: 10.1016/j.bcp.2018.01.044. Epub 2018 Jan 31. Biochem Pharmacol. 2018. PMID: 29409903 Free PMC article. Review.
-
Decline in serum cholinesterase activities predicts 2-year major adverse cardiac events.Mol Med. 2014 Feb 12;20(1):38-45. doi: 10.2119/molmed.2013.00139. Mol Med. 2014. PMID: 24395570 Free PMC article.
-
Monocyte-mediated drug delivery systems for the treatment of cardiovascular diseases.Drug Deliv Transl Res. 2018 Aug;8(4):868-882. doi: 10.1007/s13346-017-0431-2. Drug Deliv Transl Res. 2018. PMID: 29058205 Review.
References
-
- Kalow W.; Genest K. (1957) A Method for the Detection of Atypical Forms of Human Serum Cholinesterase - Determination of Dibucaine Numbers. Can. J. Biochem. Physiol. 35, 339–346. - PubMed
-
- Lockridge O.; Ladu B. N. (1978) Comparison of Atypical and Usual Human-Serum Cholinesterase - Purification, Number of Active-Sites, Substrate Affinity, and Turnover Number. J. Biol. Chem. 253, 361–366. - PubMed
-
- Darvesh S.; Hopkins D. A.; Geula C. (2003) Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 4, 131–138. - PubMed
-
- Shute C. C. D.; Lewis P. R. (1963) Cholinesterase-Containing Systems of Brain of Rat. Nature 199, 1160–1164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

