Molecular engineering of a secreted, highly homogeneous, and neurotoxic aβ dimer
- PMID: 22778868
- PMCID: PMC3369749
- DOI: 10.1021/cn200011h
Molecular engineering of a secreted, highly homogeneous, and neurotoxic aβ dimer
Abstract
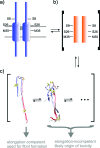
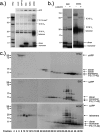
Aβ oligomers play a key role in the pathophysiology of Alzheimer's disease. Research into structure-function relationships of Aβ oligomers has been hampered by the lack of large amounts of homogeneous and stable material. Using computational chemistry, we designed conservative cysteine substitutions in Aβ aiming at accelerating and stabilizing assembly of Aβ dimers by an intermolecular disulfide bond without changing its folding. Molecular dynamics simulations suggested that mutants AβS8C and AβM35C exhibited structural properties similar to those of Aβ wildtype dimers. Full length, mutant APP was stably expressed in transfected cell lines to study assembly of Aβ oligomers in the physiological, secretory pathway and to avoid artifacts resulting from simultaneous in vitro oxidation and aggregation. Biochemical and neurophysiological analysis of supernatants indicated that AβS8C generated an exclusive, homogeneous, and neurotoxic dimer, whereas AβM35C assembled into dimers, tetramers, and higher oligomers. Thus, molecular engineering enabled generation of bioactive, homogeneous, and correctly processed Aβ dimers in vivo.
Keywords: Alzheimer’s disease; Aβ oligomers; computational chemistry; dimers; molecular engineering; neurotoxicity.
Figures
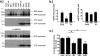
Similar articles
-
Protein engineering to stabilize soluble amyloid β-protein aggregates for structural and functional studies.FEBS J. 2011 Oct;278(20):3884-92. doi: 10.1111/j.1742-4658.2011.08295.x. Epub 2011 Sep 6. FEBS J. 2011. PMID: 21824290 Review.
-
X-ray Crystallography Reveals Parallel and Antiparallel β-Sheet Dimers of a β-Hairpin Derived from Aβ16-36 that Assemble to Form Different Tetramers.ACS Chem Neurosci. 2020 Aug 5;11(15):2340-2347. doi: 10.1021/acschemneuro.0c00290. Epub 2020 Jul 14. ACS Chem Neurosci. 2020. PMID: 32584538 Free PMC article.
-
Impact of the mutation A21G (Flemish variant) on Alzheimer's beta-amyloid dimers by molecular dynamics simulations.Biophys J. 2006 Nov 15;91(10):3829-40. doi: 10.1529/biophysj.106.090993. Epub 2006 Aug 4. Biophys J. 2006. PMID: 16891372 Free PMC article.
-
Molecular dynamics simulations of low-ordered alzheimer β-amyloid oligomers from dimer to hexamer on self-assembled monolayers.Langmuir. 2011 Dec 20;27(24):14876-87. doi: 10.1021/la2027913. Epub 2011 Nov 22. Langmuir. 2011. PMID: 22077332
-
Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer's disease.Neurol Res. 2005 Dec;27(8):869-81. doi: 10.1179/016164105X49436. Neurol Res. 2005. PMID: 16354549 Review.
Cited by
-
IPSC-Derived Neuronal Cultures Carrying the Alzheimer's Disease Associated TREM2 R47H Variant Enables the Construction of an Aβ-Induced Gene Regulatory Network.Int J Mol Sci. 2020 Jun 25;21(12):4516. doi: 10.3390/ijms21124516. Int J Mol Sci. 2020. PMID: 32630447 Free PMC article.
-
Conformational Ensembles of the Wild-Type and S8C Aβ1-42 Dimers.J Phys Chem B. 2017 Mar 23;121(11):2434-2442. doi: 10.1021/acs.jpcb.7b00267. Epub 2017 Mar 10. J Phys Chem B. 2017. PMID: 28245647 Free PMC article.
-
Synthetic dimeric Aβ(28-40) mimics the complex epitope of human anti-Aβ autoantibodies against toxic Aβ oligomers.J Biol Chem. 2013 Sep 20;288(38):27638-27645. doi: 10.1074/jbc.M113.463273. Epub 2013 Jul 11. J Biol Chem. 2013. PMID: 23846683 Free PMC article.
-
Aβ42 pentamers/hexamers are the smallest detectable oligomers in solution.Sci Rep. 2017 May 30;7(1):2493. doi: 10.1038/s41598-017-02370-3. Sci Rep. 2017. PMID: 28559586 Free PMC article.
-
Role of the N-terminus for the stability of an amyloid-β fibril with three-fold symmetry.PLoS One. 2017 Oct 12;12(10):e0186347. doi: 10.1371/journal.pone.0186347. eCollection 2017. PLoS One. 2017. PMID: 29023579 Free PMC article.
References
-
- Haass C.; Selkoe D. J. (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 8, 101–112. - PubMed
-
- Haass C.; Selkoe D. J. (1993) Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell 75, 1039–1042. - PubMed
-
- Naslund J.; Haroutunian V.; Mohs R.; Davis K. L.; Davies P.; Greengard P.; Buxbaum J. D. (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283, 1571–1577. - PubMed
-
- McLean C. A.; Cherny R. A.; Fraser F. W.; Fuller S. J.; Smith M. J.; Beyreuther K.; Bush A. I.; Masters C. L. (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 46, 860–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

