Rhodanine and thiohydantoin derivatives for detecting tau pathology in Alzheimer's brains
- PMID: 22778869
- PMCID: PMC3369744
- DOI: 10.1021/cn200002t
Rhodanine and thiohydantoin derivatives for detecting tau pathology in Alzheimer's brains
Abstract
A novel series of rhodanin (RH) and thiohydantoin (TH) derivatives were designed and synthesized for detecting tau pathology in the brains of patients with Alzheimer's disease (AD). In experiments in vitro using tau and β-amyloid (Aβ) aggregates, the TH derivative, TH2, showed high specific binding to tau aggregates. In hippocampal sections obtained from AD patients, TH2 intensely stained neurofibrillary tangles. In experiments using normal mice, [(125)I]TH2 showed good uptake (1.54%ID/g, 2 min postinjection) into and a rapid washout (0.25%ID/g, 60 min postinjection) from the brain. [(123)I]TH2 should be further investigated as a potential imaging agent for detecting tau pathology.
Keywords: Alzheimer’s disease; imaging; rhodanine; tau; thiohydantoin.
Figures
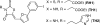





Similar articles
-
Structure-Activity Relationship Study of Heterocyclic Phenylethenyl and Pyridinylethenyl Derivatives as Tau-Imaging Agents That Selectively Detect Neurofibrillary Tangles in Alzheimer's Disease Brains.J Med Chem. 2015 Sep 24;58(18):7241-57. doi: 10.1021/acs.jmedchem.5b00440. Epub 2015 Sep 1. J Med Chem. 2015. PMID: 26327138
-
Synthesis and biological evaluation of novel styryl benzimidazole derivatives as probes for imaging of neurofibrillary tangles in Alzheimer's disease.Bioorg Med Chem. 2013 Jun 1;21(11):3356-62. doi: 10.1016/j.bmc.2013.02.054. Epub 2013 Mar 29. Bioorg Med Chem. 2013. PMID: 23601814
-
Development of a novel radioiodinated compound for amyloid and tau deposition imaging in Alzheimer's disease and tauopathy mouse models.Neuroimage. 2024 Dec 1;303:120947. doi: 10.1016/j.neuroimage.2024.120947. Epub 2024 Nov 19. Neuroimage. 2024. PMID: 39571640
-
[Development of SPECT Probes for In Vivo Imaging of β-Amyloid and Tau Aggregates in the Alzheimer's Disease Brain].Yakugaku Zasshi. 2017;137(11):1361-1365. doi: 10.1248/yakushi.17-00156. Yakugaku Zasshi. 2017. PMID: 29093372 Review. Japanese.
-
[Molecular imaging of beta-amyloid plaques in the brain].Brain Nerve. 2007 Mar;59(3):233-40. Brain Nerve. 2007. PMID: 17370649 Review. Japanese.
Cited by
-
Ligands for Protein Fibrils of Amyloid-β, α-Synuclein, and Tau.Chem Rev. 2025 Jun 11;125(11):5282-5348. doi: 10.1021/acs.chemrev.4c00838. Epub 2025 May 6. Chem Rev. 2025. PMID: 40327808 Free PMC article. Review.
-
Antibody-derived in vivo imaging of tau pathology.J Neurosci. 2014 Dec 10;34(50):16835-50. doi: 10.1523/JNEUROSCI.2755-14.2014. J Neurosci. 2014. PMID: 25505335 Free PMC article.
-
Basic Limonoid modulates Chaperone-mediated Proteostasis and dissolve Tau fibrils.Sci Rep. 2020 Mar 4;10(1):4023. doi: 10.1038/s41598-020-60773-1. Sci Rep. 2020. PMID: 32132570 Free PMC article.
-
Frontiers in Probing Alzheimer's Disease Biomarkers with Fluorescent Small Molecules.ACS Cent Sci. 2019 Feb 27;5(2):209-217. doi: 10.1021/acscentsci.8b00951. Epub 2019 Feb 14. ACS Cent Sci. 2019. PMID: 30834309 Free PMC article. Review.
-
High affinity radiopharmaceuticals based upon lansoprazole for PET imaging of aggregated tau in Alzheimer's disease and progressive supranuclear palsy: synthesis, preclinical evaluation, and lead selection.ACS Chem Neurosci. 2014 Aug 20;5(8):718-30. doi: 10.1021/cn500103u. Epub 2014 Jun 16. ACS Chem Neurosci. 2014. PMID: 24896980 Free PMC article.
References
-
- Selkoe D. J. (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766. - PubMed
-
- Mathis C. A.; Wang Y.; Klunk W. E. (2004) Imaging β-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr. Pharm. Des. 10, 1469–1492. - PubMed
-
- Nordberg A. (2004) PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 3, 519–527. - PubMed
-
- Ono M. (2009) Development of positron-emission tomography/single-photon emission computed tomography imaging probes for in vivo detection of β-amyloid plaques in Alzheimer’s brains. Chem. Pharm. Bull. (Tokyo) 57, 1029–1039. - PubMed
-
- Agdeppa E. D.; Kepe V.; Liu J.; Flores-Torres S.; Satyamurthy N.; Petric A.; Cole G. M.; Small G. W.; Huang S. C.; Barrio J. R. (2001) Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for β-amyloid plaques in Alzheimer’s disease. J. Neurosci. 21, RC189. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

