Methotrexate Increases Skeletal Muscle GLUT4 Expression and Improves Metabolic Control in Experimental Diabetes
- PMID: 22778921
- PMCID: PMC3384889
- DOI: 10.1155/2012/132056
Methotrexate Increases Skeletal Muscle GLUT4 Expression and Improves Metabolic Control in Experimental Diabetes
Abstract
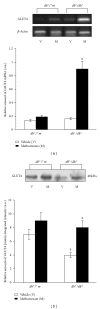
Long-term administration of 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) mimics the effects of endurance exercise by activating AMP kinase and by increasing skeletal muscle expression of GLUT4 glucose transporter. AICAR is an intermediate in the purine de novo synthesis, and its tissue concentrations can be increased, in vivo, by low doses of methotrexate (MTX) through the inhibition of the enzyme AICAR transformylase. We report here the first evidence that, in experimental type 2 diabetes, chronic treatment with low doses of MTX increases skeletal muscle GLUT4 expression and improves metabolic control. MTX (0.5 mg/kg body weight) or vehicle was administered intraperitoneally, once a week for 4 weeks, to genetically diabetic female C57BL/KsJ-m(+)/(+)Lept(db) mice (db(+)/db(+)) and their normoglycemic littermates (db(+)/(+)m). In the db(+)/db(+) mice, MTX treatment was associated with a ∼2-fold increase in skeletal muscle GLUT4 protein concentration and a >4-fold increase in GLUT4 mRNA expression (P < 0.01, all), as compared to vehicle-treated mice; no significant differences were noted in controls. MTX treatment was also associated with a significant reduction of glucose and insulin serum concentrations in diabetic mice (P < 0.001), and glucose levels only (P < 0.05) in controls. These data indicate a different route to increase skeletal muscle GLUT4 expression, through the potential inhibition of the enzyme AICAR transformylase.
Figures
Similar articles
-
Acute and chronic treatment of ob/ob and db/db mice with AICAR decreases blood glucose concentrations.Biochem Biophys Res Commun. 2002 Jun 21;294(4):798-805. doi: 10.1016/S0006-291X(02)00557-0. Biochem Biophys Res Commun. 2002. PMID: 12061777
-
α2 isoform-specific activation of 5'adenosine monophosphate-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-β-D-ribonucleoside at a physiological level activates glucose transport and increases glucose transporter 4 in mouse skeletal muscle.Metabolism. 2006 Mar;55(3):300-8. doi: 10.1016/j.metabol.2005.09.003. Metabolism. 2006. PMID: 16483872
-
5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes.Diabetes. 2003 May;52(5):1066-72. doi: 10.2337/diabetes.52.5.1066. Diabetes. 2003. PMID: 12716734
-
AMP-activated protein kinase: possible target for treatment of type 2 diabetes.Diabetes Technol Ther. 2000 Autumn;2(3):441-8. doi: 10.1089/15209150050194305. Diabetes Technol Ther. 2000. PMID: 11467346 Review.
-
Sending the signal: molecular mechanisms regulating glucose uptake.Med Sci Sports Exerc. 2004 Jul;36(7):1212-7. doi: 10.1249/01.mss.0000132387.25853.3b. Med Sci Sports Exerc. 2004. PMID: 15235328 Review.
Cited by
-
Browning of adipose tissue and increased thermogenesis induced by Methotrexate.FASEB Bioadv. 2021 Oct 7;3(11):877-887. doi: 10.1096/fba.2021-00058. eCollection 2021 Nov. FASEB Bioadv. 2021. PMID: 34761170 Free PMC article.
-
Diverse Inhibitors of De Novo Purine Synthesis Promote AICAR-Induced AMPK Activation and Glucose Uptake in L6 Myotubes.Biofactors. 2025 Jul-Aug;51(4):e70037. doi: 10.1002/biof.70037. Biofactors. 2025. PMID: 40793247 Free PMC article.
-
AICAR Improves Outcomes of Metabolic Syndrome and Type 2 Diabetes Induced by High-Fat Diet in C57Bl/6 Male Mice.Int J Mol Sci. 2022 Dec 11;23(24):15719. doi: 10.3390/ijms232415719. Int J Mol Sci. 2022. PMID: 36555360 Free PMC article.
-
Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk.J Clin Invest. 2017 Jan 3;127(1):83-93. doi: 10.1172/JCI88884. Epub 2017 Jan 3. J Clin Invest. 2017. PMID: 28045401 Free PMC article. Review.
-
Methotrexate promotes glucose uptake and lipid oxidation in skeletal muscle via AMPK activation.Diabetes. 2015 Feb;64(2):360-9. doi: 10.2337/db14-0508. Epub 2014 Oct 22. Diabetes. 2015. PMID: 25338814 Free PMC article.
References
-
- Hayashi T, Wojtaszewski JFP, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. American Journal of Physiology. 1997;273(6):E1039–E1051. - PubMed
-
- Kennedy JW, Hirshman MF, Gervino EV, et al. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999;48(5):1192–1197. - PubMed
-
- Lauritzen HP, Schertzer JD. Measuring GLUT4 translocation in mature muscle fibers. American Journal of Physiology. 2010;299(2):E169–E179. - PubMed
-
- Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Current Biology. 1994;4(4):315–324. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

